Comprehensive Lentivirus Process Solutions
Setting The Standard in Lentiviral Solutions for Gene Therapy and Gene-Modified Cell Therapy, Together
Scientists often use lentiviral (LV) vectors to deliver genes to therapeutic cells (gene-modified cell therapies) and offer potentially life-saving treatment against cancers or delivered directly to patients to treat rare genetic disorders. Because developers can use LV as a raw material or as the final therapy product, it’s critical to build robust processes that achieve consistently high viral yields with high quality.
Lentiviral Vector Gene Therapy Workflow
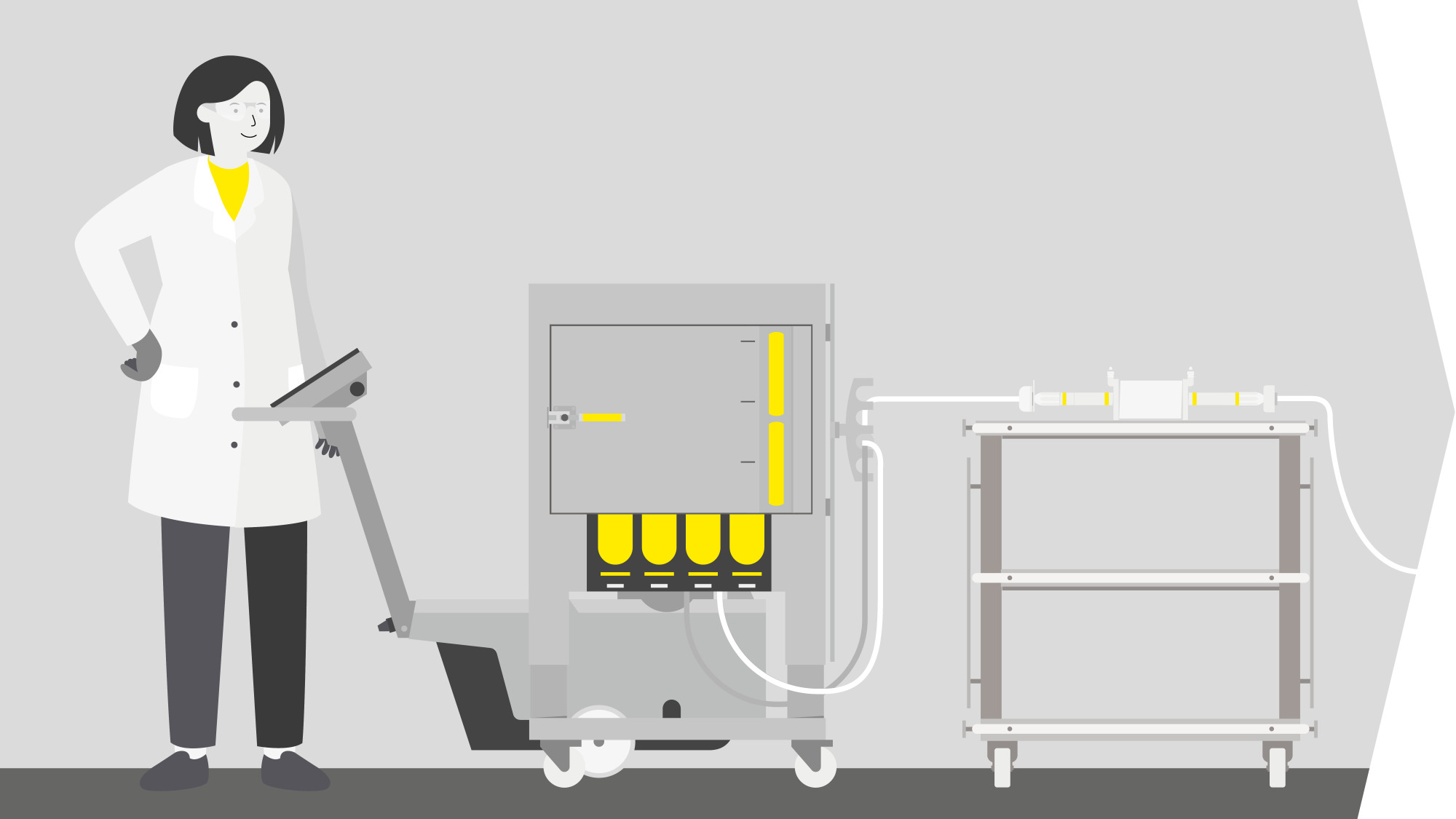
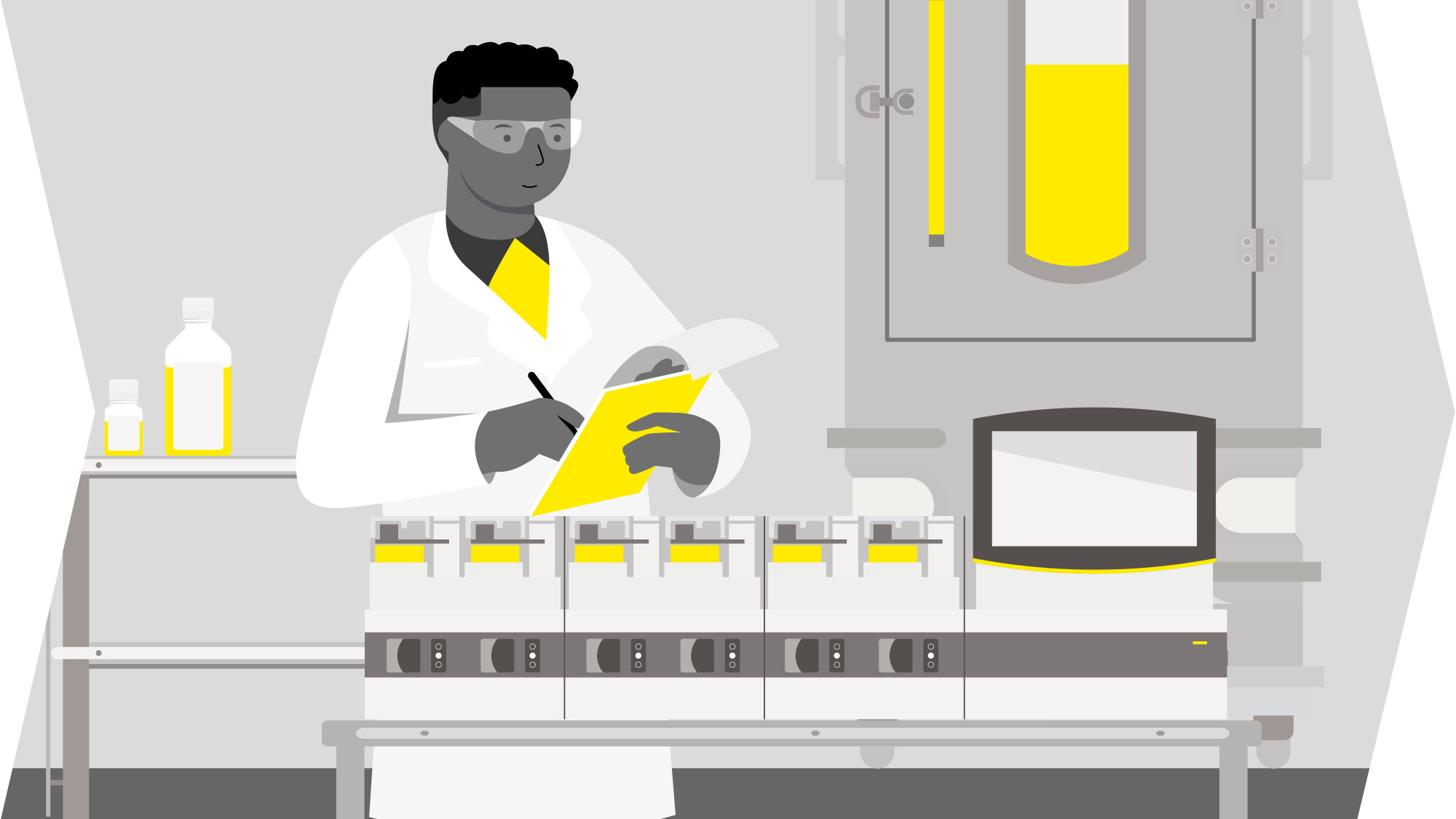
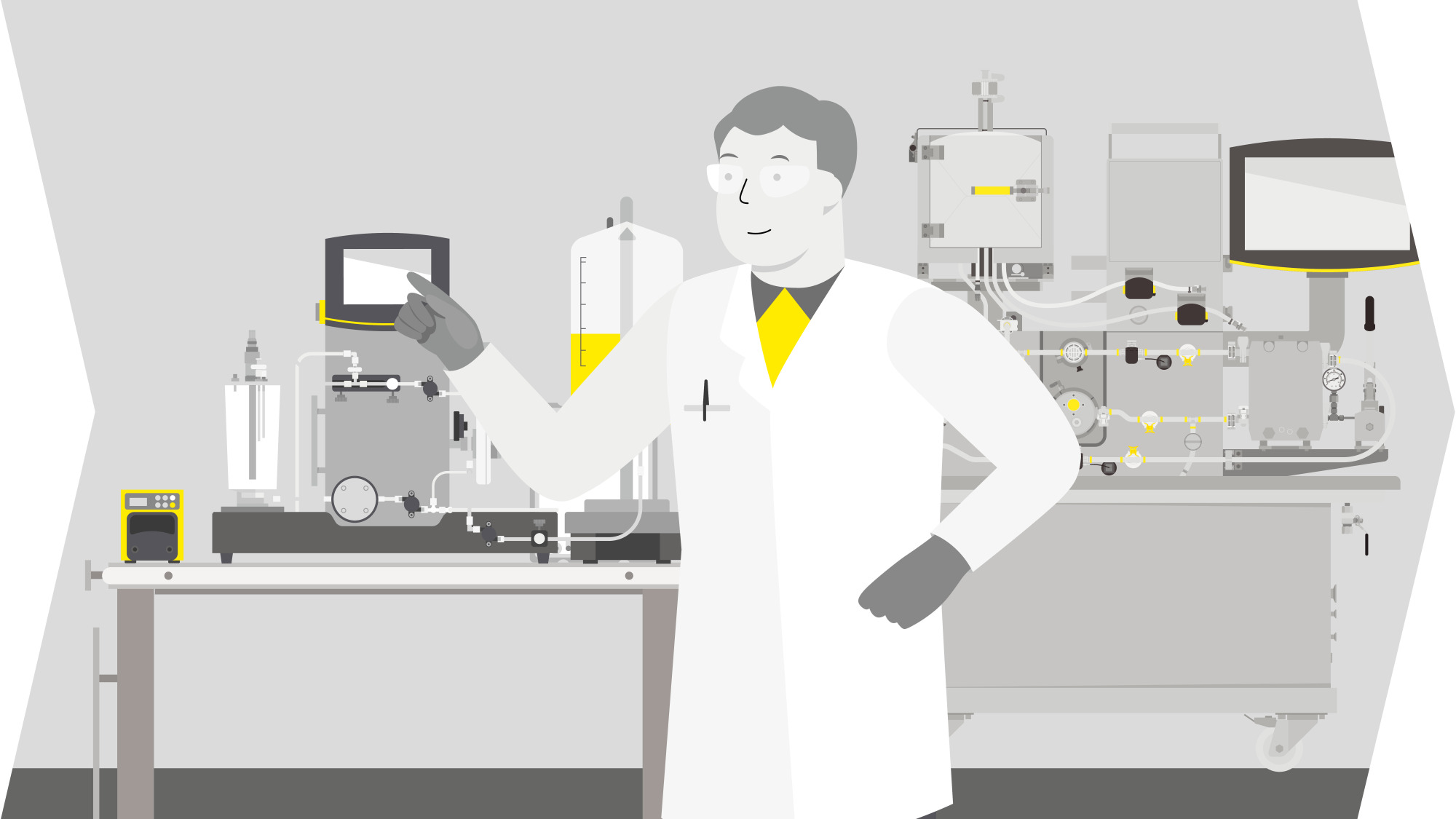
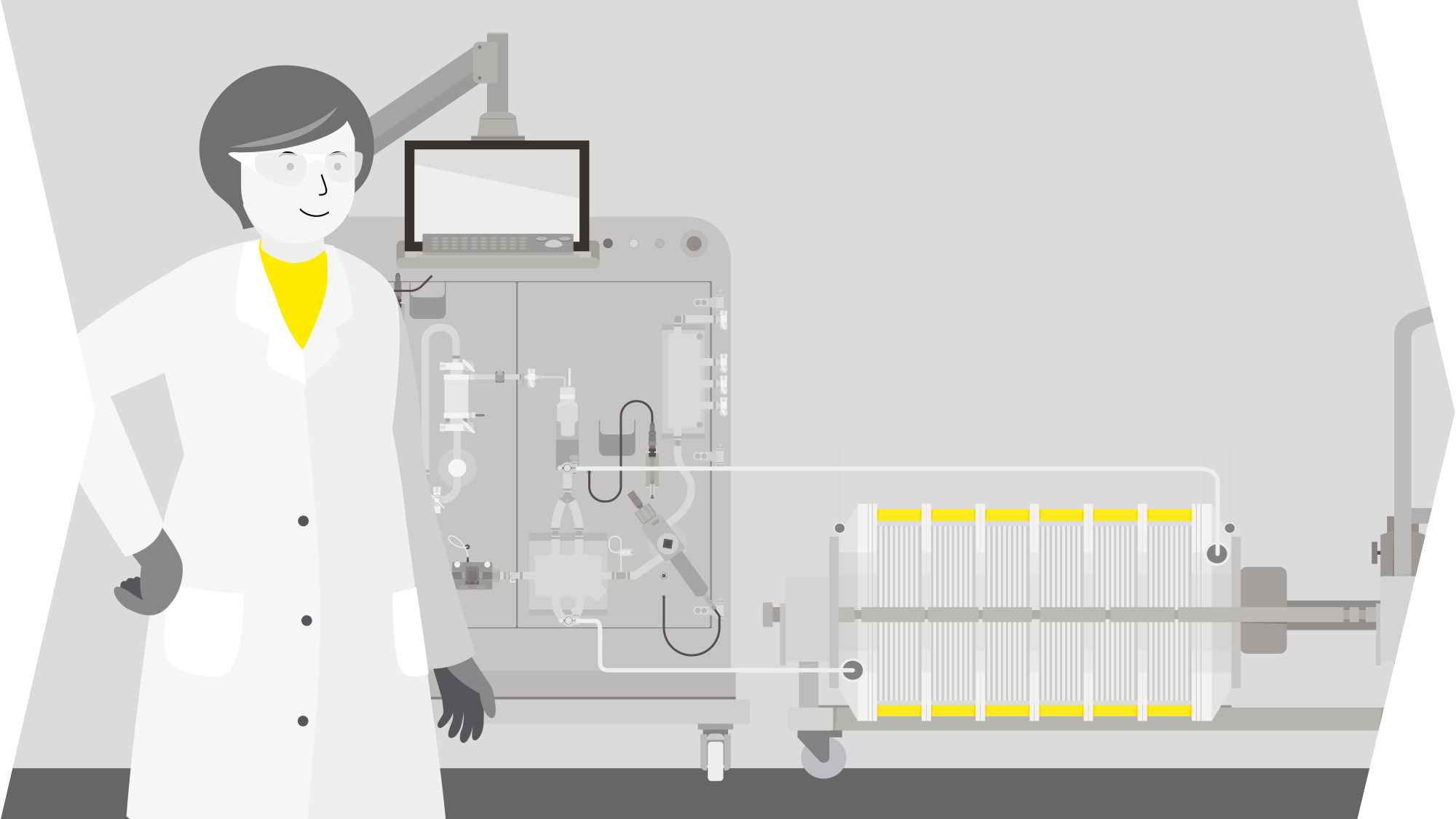
Solutions For Lentiviral Vector Production
Request Your Sample | Raw Materials and Consumables
To help find the right materials and consumables for your needs, request a sample from our portfolio.
FAQs - LV Gene Therapy
Lentiviruses are a family of viruses insert DNA into the host cells' genome upon transduction. They can permanently integrate genetic material into dividing and nondividing cells. Lentiviral vectors are produced from a culture of packaging cells, which scientists then transfect with plasmids. The vector-producing cells are then expanded in culture and purified.
Lentiviruses are transducing, dividing, and nondividing cells, and enable long-term expression by integrating into the cell genome. They have a large payload of 9.7 kb. Lentivirus is unique in its ability to infenct nondividing cells, and has high transduction toward targets like T-cells.
BSL2. Scientists can't perform viral clearance on lentiviruses using a filter, so single-use and closed systems are prefered. Scientists need to control raw materials and filter them to remove virus. Removing impurities like DNA and HCP is difficult because lentiviruses are large and sensitive, making downstream process challenging.