Comprehensive Stem Cell Therapy Solutions
Setting the Standard in Stem Cell Therapy, Together
Stem cell therapies are advancing regenerative medicine and offering treatment options for diseases once deemed incurable. Since the cells themselves are the drug product, reliably isolating, expanding, and differentiating stem cells to target phenotypes is critical for getting the cell counts required for clinical applications.
Bring your therapy to market with end-to-end solutions that deliver reproducible performance and scalability with a simplified path to regulatory approvals.
Every step of the way, Sartorius helps you make a difference by making the difference in your process – from discovery to process development to clinical and commercial manufacturing. Together, we're setting the standard in stem cell therapy.
Stem Cell Therapy Workflow
Stem Cell Therapy Products
Request Your Sample | Raw Materials and Consumables
To help find the right materials and consumables for your needs, request a sample from our portfolio.
Stem Cell R&D Solutions
Stem Cell Research and Development Solutions
FAQs - Stem Cells Therapy
Stem cell therapy manufacturing is the process of producing a stem cell therapy medicinal product. Stem cell therapies have applications in regenerative medicine, autoimmune diseases, and other rare diseases.
Gene therapy can be used for in vivo treatment, where the treatment is injected directly into the patient. In ex vivo treatment, also known as gene-modified cell therapy, the cells are extracted, modified, and then reinjected into the patient. Stem cell therapy is often referred to as regenerative medicine. Cells like mesenchymal stem cells, induced pluripotent stem cells, and hematopoietic stem cells are all used for gene therapies.
Today, scientists are developing cutting-edge stem cell therapies to target conditions like osteoarthritis, type 1 diabetes, Parkinson's disease, and more. There are already approved stem cell therapies to treat cardiovascular, musculoskeletal, and metabolic disorders.
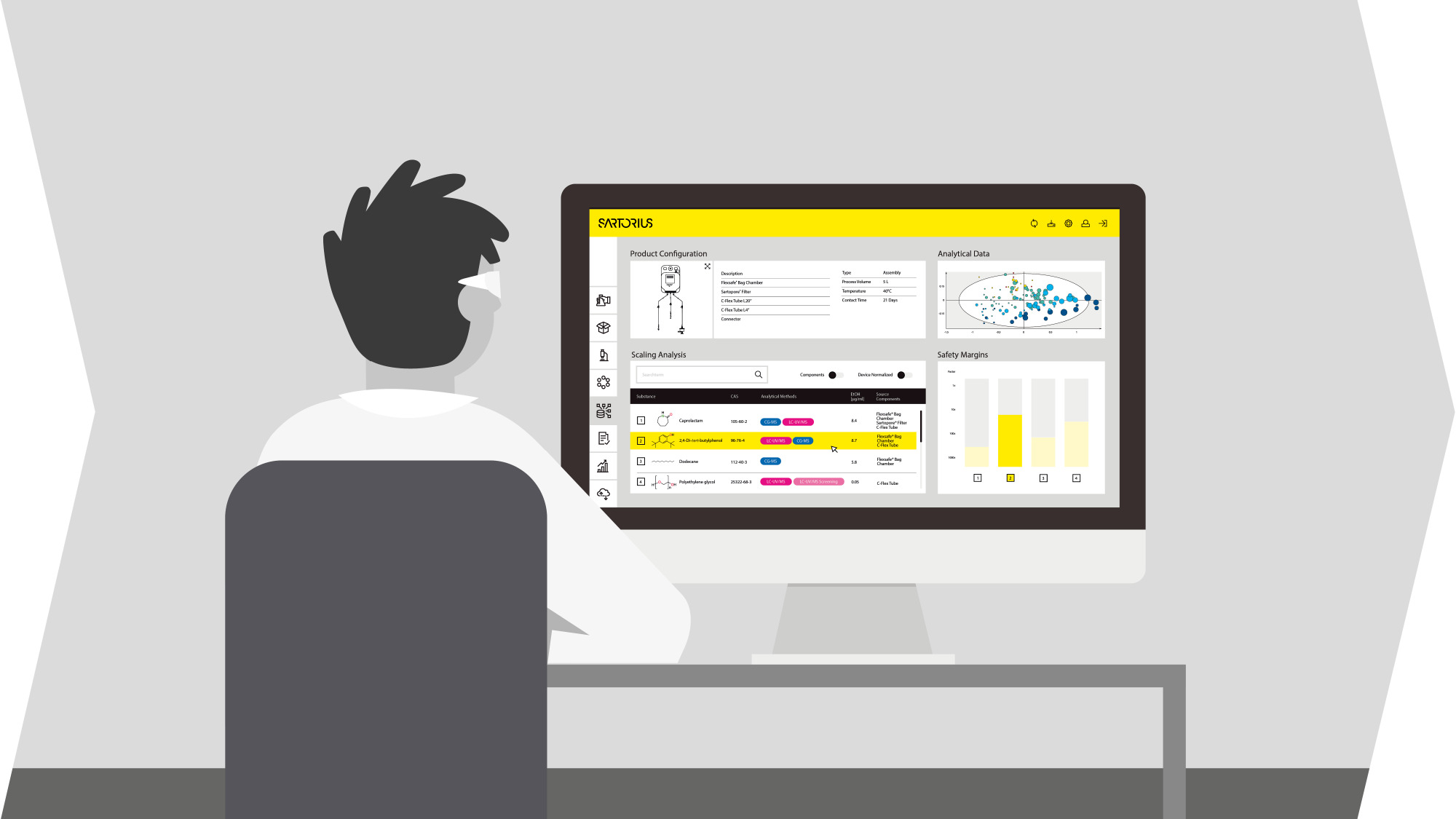
Sartorius provides GMP and automated tools with comprehensive analytics software. Our automated bioreactors use a design of experiments approach to accelerate optimization. We also provide high-quality raw materials suitable for GMP-grade manufacturing, available in a variety of sizes and packaging. Our goal is to enable stem cell therapy developers to achieve reproducible performance at all scales.