10x Faster Aseptic Filling with Single-Use Plug and Play Setup
Minimize filling time with an efficient aliquoting solution that pumps fluid to 10 bags simultaneously.
The Linkit® AX Aliquoting Solution is ideal for aliquoting small quantities of process fluids - fast, evenly, and reproducible batch-to-batch. The plug & play concept, combined with ready-to-use patented manifold design, minimizes operator effort and error and consistently distributes fluid between the 10 bags with an accuracy of ±5%. The solution is designed to address the pain points of manual filling and aliquoting applications without the need to heavily invest in big and complex equipment.
The Smart Aliquoting Solution
Watch how aliquoting can be simplified with the Linkit® AX Solution
Fast filling performance with a unique flow-through fluid design of the main hub.
QApp guides the operator during a filling operation, ensuring batch-to-batch repeatability.
Fully assembled, compact and small footprint solution - no need to handle bulky and complicated traditional manifolds.
Full end-to-end traceability in compliance to FDA directive 21 CFR part 11 and EU Annex 11.
Simplify Aliquoting
There is no product like the Linkit® AX in the market today
Aliquoting is a common task in bioprocessing - often done manually, with all the pain points that accompanies the use of traditional bag manifolds: bulky, difficult to handle & manipulate and prone to operator error.
Fully automated aliquoting systems require considerable capital investment and are more suited to drug substance and drug product filling applications.
The Linkit® AX aliquoting system is the first simultaneous filling solution, offering fast filling with a distribution accuracy of ±5.1% and not requiring an initial high capital expenditure cost.
Attribute | Traditional Bag Manifold | Linkit® AX | High-End Automated Filling System |
|---|---|---|---|
CAPEX | Low | Medium | High |
Filling | Sequential | Simultaneous | Sequential |
Set-up | Manual | Plug and Play | Manual |
Automation | None | Semi | Full |
Operator Effort | High | Low | Med-Low |
Filling Time for 50 L Batch | 6 Hours | 30 min | 1 - 2.5 Hours |
Manual Aliquoting vs. The Linkit AX® Solution: A Side by Side Comparison
Watch our video to see the innovative ways Linkit AX® is designed to simplify and speed up your aliquoting process.
Featured Assets
Linkit® AX Aliquoting Solution
Have a Closer Look
The Linkit® AX Aliquoting System consists of four elements:
- Linkit® AX Single-Use Assembly
- Pump to fill the bags
- Cubis® II balance with QApp software to control the pump and the filling
- Purge stand to position the purge system and inlet filling line correctly
Linkit® AX Aliquoting Solution

Request Your Free Demonstration of Linkit® AX
Integrating the Linkit® AX Into Your Process
The Linkit® AX Single-Use assembly is available with different configurations dependent on process needs.
The main processing unit has a fixed design, however certain options are available to choose from and inlets | outlets can be configured to meet and easily integrate any specific process requirements.
Linkit® AX
- Select Options
▪ Flexsafe® bag: 150, 250, 500 or 1,000 mL
▪ Purge system: Sartopore® Air MidiSart® 0.2 μm or 500 mL Flexsafe® bag - Inlet | Outlet Process Configurations
▪ Inlet Linkit® AX to feed container
▪ Outlet 1 (and 2 on 1,000 mL Flexsafe® bag)
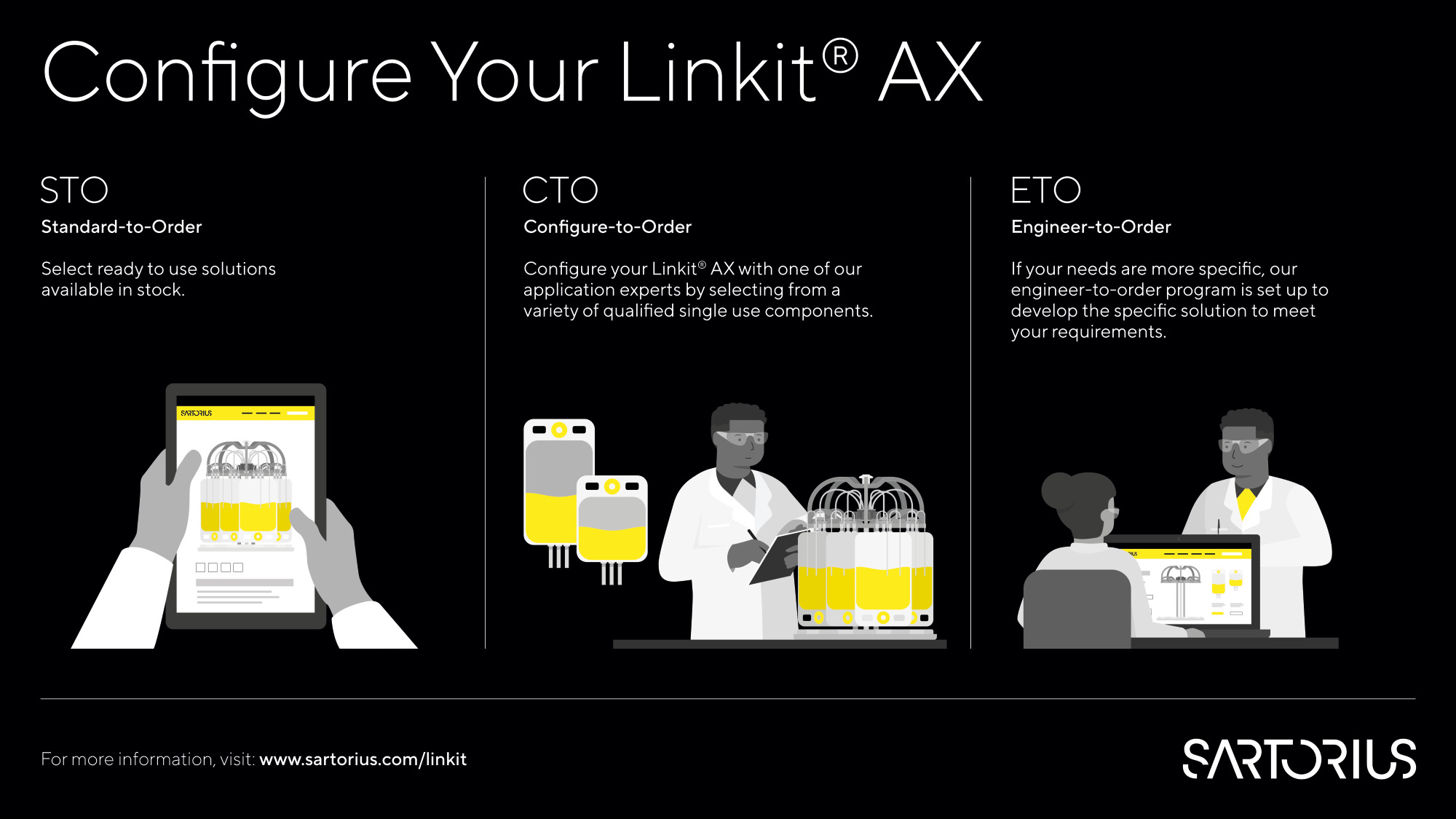
Sartorius offers a range of pre-designed Linkit® AX single-use assemblies. If the standard-to-order designs do not meet the process requirements, our extensive component library in the configure-to-order space or our design experts can tailor solutions to meet them.
Learn More About Linkit® AX
FAQs – Linkit® AX Aliquoting System
150, 250, 500 and 1,000 mL Flexsafe® 2D Bags.
Yes, the Cubis® II Balance keeps 21 CFR part 11 compliant digital batch records that can easily be exported.
10 Flexsafe® bags.
Opta® SFT, AseptiQuik®, ReadyMate, Kleenpak™ HT, Kleenpak™ Presto and plugs on TPE tubes, however if your process requires another type of connection, our design experts are here to assist you.
We've Got You Covered - Meeting cGMP Guidelines
Linkit® AX single-use assemblies are assembled in ISO 7 certified cleanrooms in accordance with applicable cGMP guidelines. Each system undergoes a variety of in-process quality inspections including 100% visual inspection and 100% leak testing (pressure decay) of the main distribution assembly hub and Flexsafe® bag chambers. To support you in your regulatory needs, specific documentation is available upon request:
|
|
In case additional testing is required, you can request your validation study via Confidence® Validation Services.