Comprehensive Immune Cell Therapy Solutions
Setting the Standard in Immune Cell Therapy, Together
Immune cell therapies are a major advancement in the fight against blood cancers. While most of today’s commercialized therapies use harvested patient cells, increasing demand calls for allogeneic solutions — and developers need robust processes that can scale up, maintain potency, and reduce cost per dose.
Bring your immune cell therapy to market faster and more affordably with end-to-end solutions that deliver reproducible performance and scalability with a simplified path to regulatory approvals.
Immune Cell Therapy Workflow
Spotlight on Success: University College London (UCL)
Overcoming CAR-T Manufacturing Challenges, Together.
Bringing CAR-T therapies to more patients depends on overcoming one of the field’s biggest challenges: manufacturing. In this collaborative story with Dr. Qasim Rafiq’s lab at University College London (UCL), we set out to explore a smarter, more scalable way to grow and harvest CAR-T cells. Through shared expertise and innovative tools, the team demonstrated how process optimization and automation can make the promise of CAR-T manufacturing more achievable, efficient, consistent, and ready to scale.
Immune Cell Therapy Products
Request Your Sample | Raw Materials and Consumables
To help find the right materials and consumables for your needs, request a sample from our portfolio.
FAQs - Immune Cell Therapy
Immune cell therapy manufacturing is the process of producing a immune cell therapy medicinal product. Immune cell therapies have applications primarily in oncology and autoimmune diseases.
The most prominent types of immune cell therapies are T cells that are genetically engineered to express chimeric antigen receptors (CARs) or T cell recepters (TCRs), and tumor infiltrating lymphocytes (TILs). The other cell types include natural killer (NK) cells and dendritic cells (DCs).
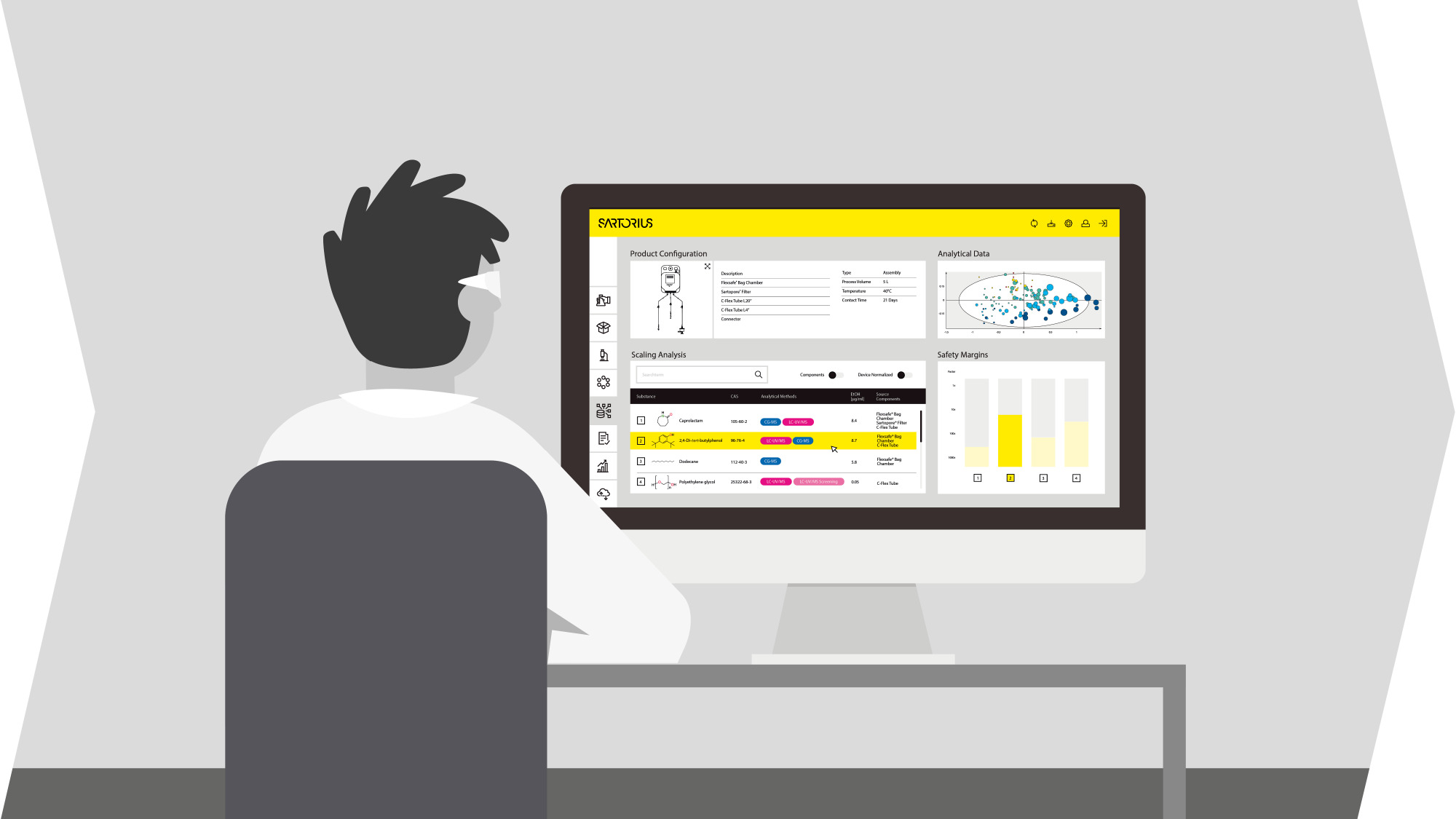
Sartorius provides GMP and automated tools with comprehensive analytics software. Our automated bioreactors use a design of experiments approach to accelerate optimization. We also provide high-quality raw materials suitable for GMP-grade manufacturing, available in a variety of sizes and packaging. Our goal is to enable immune cell therapy developers to achieve reproducible performance at all scales.