Antibody Drug Conjugates (ADCs)
Antibody Drug Conjugates (ADCs) are a transformative class of next generation biopharmaceuticals that are redefining the landscape of cancer treatment and other difficult to treat indications. ADCs represent a synergistic combination of the targeting capabilities of monoclonal antibodies with the cell-killing power of cytotoxic drugs, the payload. Researchers are currently developing novel design of ADCs to further improve their efficacy and reduce off tumor toxicities.
The latest generation of ADCs show promising therapeutic outcomes and have led to the rapid growth of this precision immunotherapeutic class. New advancements in conjugation and linker technologies are at the forefront of next-generation ADC development, broadening the scope of oncology with targeted drugs for difficult-to-treat cancers like solid tumors, ovarian, pancreatic, glioblastoma, prostate cancer and beyond.
Current development challenges include:
- Complexity: The complex makeup and combination of Mechanism of Action (MoA) of ADCs require orthogonal in vitro characterization and cell-based assays with high accuracy and precision
- Linker Stability: Developing chemically stable linkers that are cleavable within target cells is essential to ensure the selective release of the cytotoxic payload
- Cytotoxic Payload: The potency and tolerability of the cytotoxic drugs used in ADCs must be optimized to improve the therapeutic index and reduce side effects.
Sartorius is addressing these challenges with innovative analytical techniques, cell-based assays, and 3D advanced cell models that streamline the research and development of ADC candidates:
- Critical quality attributes (CQAs) with combined platforms
- In-depth analysis of ADC activity and specificity
- Insightful assessment of different mechanism of action (MoA)
ADCs Mechanism of Action
Researchers aim to create powerful workflows for ADC characterization that can uncover differences in binding and functional Mechanism of Action (MoA), which can inform and enhance antibody discovery processes. The MoA for most FDA-approved and pre-/clinical ADC drug candidates typically follows these steps:
Targeting: The ADC circulates in the bloodstream until it encounters a cancer cell with the appropriate antigen on its surface. The antibody component of the ADC binds to the antigen.
Internalization: After binding to the antigen, the ADC-antigen complex is internalized by the cancer cell through a process called receptor-mediated endocytosis.
Release of Payload: Inside the cell, the linker is cleaved, often in response to the acidic environment of the lysosomes or by specific enzymes, releasing the cytotoxic drug.
Cell Death: The released drug then interferes with a critical cellular process, such as DNA replication or protein synthesis, leading to the death of the cancer cell.
ADC R&D Workflow
Featured Products for Antibody Drug Conjugates (ADCs)
iQue® High-Throughput Screening (HTS) Cytometry Platform
The iQue® HTS Cytometry Platform is a high-throughput, suspension cell and bead analysis platform for multiplexed analysis of cell phenotype and function in a single well allowing for streamlined workflow and highly informed date sets. The iQue® 21 CFR Part 11 Software Module ensures easy transitions from research lab towards regulated laboratories.
Comprehensive functional profiling: Antibody-Dependent Cellular Cytotoxicity (ADCC) studies including quantification of critical Natural Killer (NK) cell activation markers
- Complement-Dependent Cytotoxicity (CDC) assay
- Live-cell antibody binding potency assay
- ADC cytotoxicity, quantification of cell death
- Quantification of cytokines involved in NK killing mechanism
- High-throughput analysis of bystander activity
Explore High-Throughput Screening (HTS) by Cytometry Read Application Note
Octet® Label-Free Protein Analysis Platforms
Octet® Biolayer Interferometry (BLI) and Surface Plasmon Resonance (SPR) platforms measure protein-protein interactions in parallel, without the use of detection agents. These robust approaches enable fast, real-time characterization of binding and activity of ADC to target receptors, even in complex and unpurified samples.
- Rapid analysis of ADCs binding to target antigens, i.e. HER2
- In-depth characterization of kinetic analysis of target and FcγR binding
- Direct quantitation of cytokines
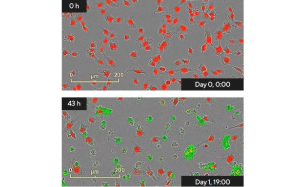
Incucyte® Live-Cell Analysis System
The Incucyte® Live-Cell Analysis System is designed to efficiently capture cellular changes where they happen - in the incubator. It enables in-depth functional and phenotypic assessment of several cell types and cellular interactions such us immune cell activation, cell health and proliferation.
Incucyte® 21 CFR Part 11 Software Module offers comprehensive security and electronic record keeping functionality.
- ADC internalization
- Target cell killing
- Cell proliferation, health, apoptosis,
- Cell cycle analysis
- ADC cytotoxicity and ADCC /ADCP activity assays - also in spheroid models
- Bystander killing - also in spheroid models
Build a comprehensive in vitro functional and phenotyping profile of ADCs candidates with combined live-cell and biolayer interferometry platform.
Explore Live Cell Imaging & Analysis
Learn more about evaluating ADCs in vitro using 3D tumor spheroid models
CellCelector Automated Cell Selection and Retrieval Platform
The CellCelector Flex Platform is a fully automated cell imaging and picking system developed for screening, selection and isolation of single cells, clones, clusters and adherent colonies. The CellCelector Flex is unique, as it combines a powerful high content imaging system with a fully automated cell-picking platform. It can process hundreds of thousands of cells in parallel to identify and select optimal clones that secrete high levels of the desired antibody within 8 hours.
Simplifying Antibody Discovery with Automation:
- Single Cell Cloning for CLD
- Single Plasma B-Cell Secretion Screening and Recovery
- Hybridoma and CHO Clone Picking
Lab Filtration and Purification
Fast and easy macromolecule purification for your R&D projects, using process-ready Sartobind® membranes.
Sartobind Protein A Lab
Rapid affinity purification for IgGs and mAbs, with a process-ready protein A membrane.
Sartobind IEX Lab
Rapid, economical IEX for parallel screening and small-scale contaminant removal after affinity chromatography.
- Out-of-the-Box Flexibility Connect directly to a syringe, pump or FPLC system with no need to order expensive adapters separately – everything you need is already included.
- High Yields in Seconds Rapid transport of even the largest molecules to the functionalized surface through convective flow, reduce cycle times and maximize productivity.
- Process-Ready Technology Based on the industry-leading Sartobind® platform to eliminate uncertainty and minimize optimization requirements when transitioning to production.
Sartorius Antibody Drug Conjugates (ADCs) Solutions Offer:
Incucyte®, iQue®, and Octet® BLI instruments all include 21 CFR Part 11 Software Module enable electronic record keeping and traceability, user management as well as electronic signatures. This supports the easy transition of assay from the R&D phase towards clinical pipelines and commercial manufacturing.
Combination of optimized workflows allow for data generation across multiple platforms with ease, facilitating broader profiling of many different ADC characteristics. For example, in addition to ADCC activity, the iQue® also quantifies other critical activation markers and cytokines (CD25, CD69, IFNγ. and Granzyme B), which, when combined with Octet® binding affinity data, can provide researchers with useful biophysical and functional information during ADCs development.
Enjoy walk-away convenience as images are automatically acquired and analyzed. Multiplex measurements in 96- and 384-well assay formats.
Frequently Asked Quesetions
ADCs are targeted cancer therapies that combine the specificity of monoclonal antibodies with the potency of cytotoxic drugs. They are designed to selectively deliver these potent agents to cancer cells, thereby minimizing the systemic toxicity associated with traditional chemotherapy.
An ADC consists of three main components:
Antibody: The antibody is selected for its ability to bind specifically to an antigen that is expressed on the surface of cancer cells. The antigen should ideally be unique to cancer cells or at least be significantly overexpressed on them compared to normal cells. This ensures that the ADC targets and binds to the cancer cells with high specificity.
Cytotoxic Drug (Payload): The drug attached to the antibody is typically a highly potent cytotoxic agent that can kill cancer cells. These drugs are often too toxic to be used alone, but when delivered directly to cancer cells by the antibody, they can be used effectively at lower, less toxic doses.
Linker: The linker is a chemical or peptide bond that connects the cytotoxic drug to the antibody. It is designed to be stable in the bloodstream to prevent premature release of the drug, which could cause toxicity to healthy tissues. However, once the ADC is internalized by the cancer cell, the linker is cleaved by cellular processes, releasing the cytotoxic drug to exert its cell-killing effects.
ADCs are indeed considered biologics. Biologics are a category of drugs that are derived from living organisms or contain components of living organisms. ADCs are complex molecules composed of an antibody linked to a biologically active drug or cytotoxic compound. The antibody part of the ADC is a protein that is produced using biotechnological methods in living cells, which classifies it as a biological product.
The U.S. Food and Drug Administration (FDA) regulates ADCs under the Center for Drug Evaluation and Research (CDER) as biologics, and they are subject to the regulatory pathways that apply to biological products, including the Biologics License Application (BLA) process.
ADCs combine the targeting capabilities of monoclonal antibodies (which are a type of biologic) with the cancer-killing ability of cytotoxic drugs, allowing for more precise targeting of cancer cells while minimizing the impact on healthy cells. This makes them a powerful tool in the treatment of certain types of cancer.
ADCs are complex pharmaceuticals designed to deliver cytotoxic drugs specifically to targeted cancer cells. The production of ADCs involves several key steps:
Antibody Production:
The process begins with the generation of monoclonal antibodies (mAbs) that are specific to antigens expressed on the surface of cancer cells. These antibodies are produced by immune cells that have been cloned from a single parent cell, ensuring specificity and uniformity.
The production of mAbs typically involves the use of mammalian cell culture systems, such as Chinese hamster ovary (CHO) cells, which are engineered to produce the desired antibody.
Cytotoxic Drug and Linker Synthesis:
The cytotoxic drug, also known as the "payload," is a potent agent that can kill cancer cells. It is usually too toxic to be administered alone.
A linker molecule is chemically synthesized to connect the cytotoxic drug to the antibody. The linker must be stable in the bloodstream to prevent premature release of the drug but should release the drug once inside the targeted cancer cell.
Conjugation:
The antibody is chemically conjugated to the cytotoxic drug via the linker. This step is critical and requires precise control to ensure that the correct number of drug molecules are attached to each antibody molecule. This ratio is known as the drug-to-antibody ratio (DAR).
Conjugation methods vary, but common approaches include the use of cysteine or lysine residues on the antibody to form a stable chemical bond with the linker.
Purification:
After conjugation, the ADC mixture contains species with different DARs, as well as unconjugated antibodies and free drugs. The mixture is purified to isolate the ADC with the desired DAR and to remove impurities.
Techniques such as hydrophobic interaction chromatography (HIC), size-exclusion chromatography (SEC), or affinity chromatography may be used for purification.
Quality Control and Characterization:
The final ADC product undergoes rigorous quality control testing to ensure its purity, stability, and biological activity. This includes tests for aggregation, free drug content, and the functionality of the antibody.
Characterization techniques such as flow cytometry, mass spectrometry, high-performance liquid chromatography (HPLC), and Enzyme-Linked Immunosorbent Assay (ELISA) may be used to analyze the ADC's properties.
Formulation and Packaging:
The purified ADC is formulated into a stable pharmaceutical preparation, which may involve the addition of stabilizers or excipients.
The final product is filled into vials or other suitable containers under aseptic conditions and is then packaged for distribution.
Yes, ADCs are considered a form of targeted therapy. They are designed to specifically target and kill cancer cells while sparing healthy cells, which is a hallmark of targeted cancer therapies. The monoclonal antibody component of an ADC selectively binds to antigens that are typically overexpressed on the surface of cancer cells, delivering the cytotoxic drug directly to the tumor. This targeted approach aims to improve the efficacy of the treatment and reduce the side effects associated with traditional chemotherapy.
ADCs have been developed to treat a variety of cancer types. The applicability of an ADC to a specific cancer depends on the presence of a targetable antigen that is expressed on the surface of the cancer cells. Some of the cancer types that ADCs have been approved for or are being investigated to treat include:
- Breast Cancer: ADCs such as trastuzumab emtansine (Kadcyla) target HER2-positive breast cancer.
- Lymphoma: Brentuximab vedotin (Adcetris) is used to treat Hodgkin lymphoma and systemic anaplastic large cell lymphoma.
- Leukemia: Gemtuzumab ozogamicin (Mylotarg) targets CD33-positive acute myeloid leukemia (AML).
- Bladder Cancer: Enfortumab vedotin (Padcev) is used for urothelial carcinoma, the most common type of bladder cancer.
- Lung Cancer: There are ADCs in development for non-small cell lung cancer (NSCLC) targeting specific antigens.
- Gastric Cancer: Trastuzumab deruxtecan (Enhertu) is used for HER2-positive gastric or gastroesophageal junction adenocarcinoma.
- Multiple Myeloma: There are ADCs being investigated for multiple myeloma, targeting antigens such as BCMA (B-cell maturation antigen).
The development of ADCs is an active area of research, and many more ADCs are in clinical trials for a range of other cancers. The success of an ADC in treating a particular type of cancer depends on the identification of suitable antigens that are highly expressed on cancer cells and the ability to target those antigens with an antibody that can deliver a cytotoxic payload effectively.