Simplifying the Complexity of Proving Biosimilarity
Biosimilars are biological medicines that are extremely similar to already-approved biopharmaceutical drugs (originator or reference medicinal product (RMP)). Biosimilars can be manufactured when the original product’s patent expires.
A comprehensive analytical data package is the foundation of bringing a biosimilar to market. It is critical that drug developers prove there are no clinically meaningful differences between the biosimilar and its RMP in terms of the safety, purity and potency of the product.
Discover how Sartorius solutions can simplify this complex process and help bring your biosimilar to market faster.
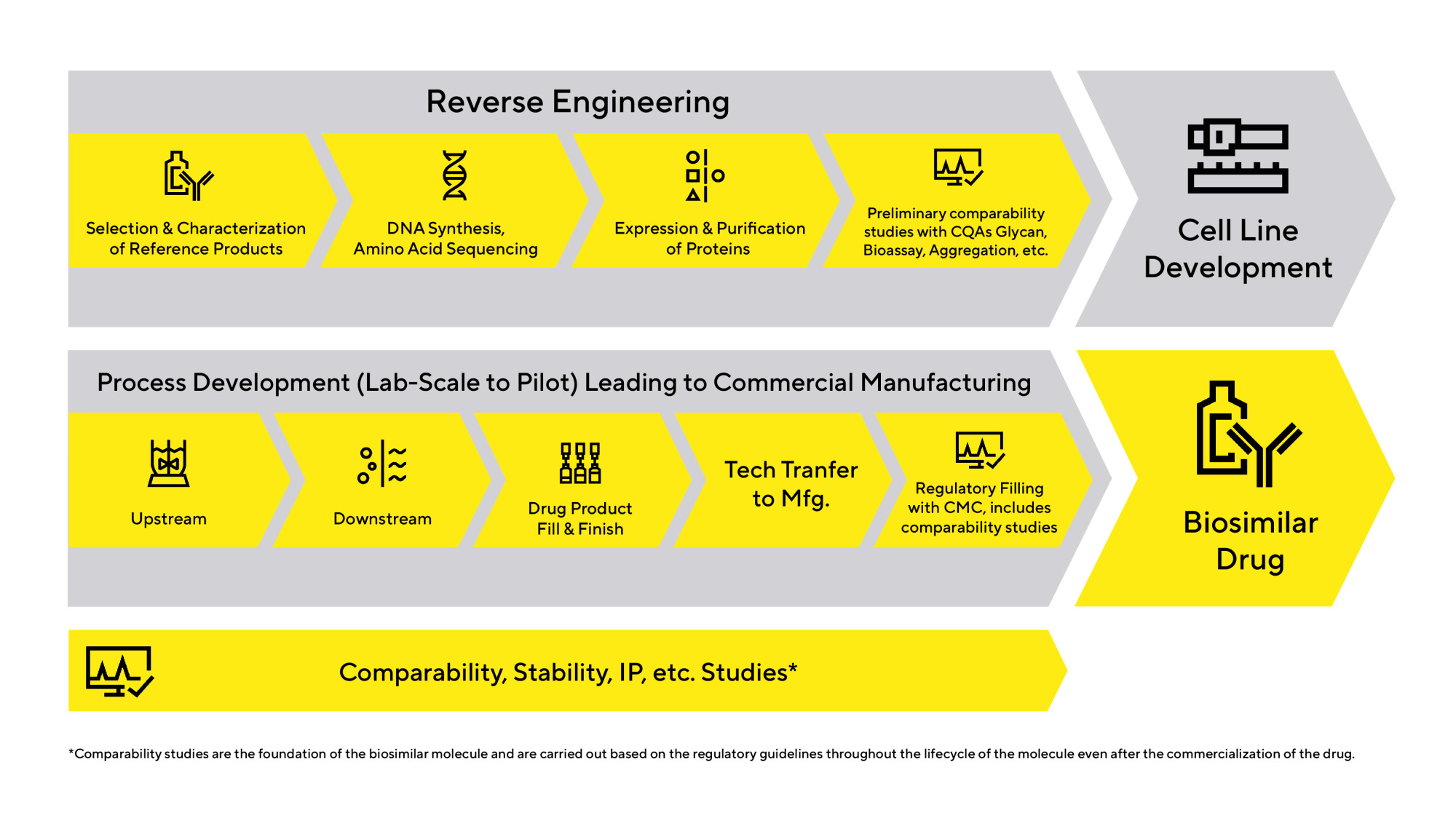
Biosimilars Development Workflow
Product Highlights for Biosimilar Development
iQue® 5 High-Throughput Screening (HTS) Cytometry Platform
The new iQue® 5 is designed for speed, simplicity and scalability. Perform simultaneous phenotype and functional analysis of secreted cytokines. Drive progress in automated antibody discovery and screening, T-cell engineering and immuno-oncology.
Don’t miss the chance to experience:
- Next-level performance
- Effortless operation
- Powerful and intuitive software
- Simplified, extended runs
Octet® BLI Label-Free Detection for Biomolecular Interactions Analysis
Gain unprecedented time and cost savings for kinetics, affinity and concentration assays.
Incucyte ® Live-Cell Analysis System
Allows visualizations and direct quantitation of the full timecourse of biology like cell activation, proliferation and vitality.
Vivaspin® and Vivaflow® Ultrafiltration Devices
With a wide range of process volumes and a broad choice of membrane materials and molecular weight cut-offs, Vivaspin® and Vivaflow® serve as the perfect tools for every lab scale protein, nucleic acid, nanoparticle or virus concentration step.
HPLC Sample Preparation
Semi-automatic preparation and documentation of highly reproducible HPLC calibration standards with the Cubis® MSA dosing system.
Cubis® II: 100% Configurable Laboratory Balance
With completely configurable hardware, software and connectivity, Cubis® II offers a high-performance balance that will align with your unique demands and compliance requirements.
Cubis® II MPS116S Pipette Calibration System
With just one device instead of three, you´ll optimize valuable lab space, cut maintenance costs, and simplify setup at customer sites. It´s a single, powerful system for fast, reliable, and fully ISO 8655-compliar calibration for single-cannel pipettes.
Biosimilar Testing Services
As the leading experts in biosimilars, we understand demonstrating your biosimilar has fingerprint-like similarity to the reference product is a complex process. With our complete range of testing and development services from biosimilar cell line development, off-the-shelf and custom assays, to biosafety testing and cell bank manufacturing - and our unrivalled expertise in the field - we are your complete solution provider.
Sartorius Biosimilar Development Solutions Offer:
Analyze multiple parameters with the same assay:
High throughput, multiplex assay that measures ADCP via co-localization of encoding dye-labeled target cells with CD14 positive effector cells using a simplified, streamlined workflow with iQue® 5 High-Throughput Screening (HTS) Cytometry Platform
Glycan screening combined with titer measurement with Octet® BLI
Visualization and direct quantification changes in morphology, movement and spatial orientation of cancer and immune cells over days and weeks
Conserve precious sample through multiplexing – analyze multiple parameters from the same well from low sample volume (as little as 1uL minimum sample volume requirement) with iQue® 5 High-Throughput Screening (HTS) Cytometry Platform.
Lower hands-on manipulation and sample consumptions; more in-depth analysis method compared to ELISA using Octet® BLI.
iQue® 5 High-Throughput Screening (HTS) Cytometry Platform enables rapid, high-throughput walkaway plate sampling and automated analysis and reporting.
Incucyte® Live-Cell Analysis System automatically acquires and analyzes phase and fluorescent images of cells around the clock.
Frequently Asked Questions
It does not require cells be removed from the incubator and lifting, washing or labeling with perturbing (and expensive) antibodies is not necessary. This eliminates a wide range of potential assay artifacts and affords significant additional biological insight.
To compare the antibody-dependent (tumor) cell killing (ADCC) of trastuzumab and potential biosimilar mAbs, Incucyte® CLCA assays were assembled with co-cultures of HER2-positive SKOV-3 ovarian cancer cells and peripheral blood mononuclear cells.