Customized Cell Line Development Solutions for Your Unique Processes
A Tailored Approach to Cell Line Development
Bringing a biologic to market depends on smart cell line development strategies. You need to balance the control and speed of an in-house solution with the convenience and flexibility of outsourcing. Sartorius offers a suite of innovative products and services that can be tailored to your operation. Whether you need instruments, consulting, or development and testing services, partnering with Sartorius can accelerate your cell line development and ensure a smooth transition to commercial manufacturing.
Choose just the right products and services to complement your core strengths
Leverage the right people and products when and where you need them
A single-vendor approach simplifies management and administrative activities
Accelerate Your Cell Line Development at Every Step
Producing biologics is complex, and cell line development is no exception — tight timelines, constant cost pressure, and time-to-market are top of mind. Starting with customizable solutions from Sartorius gives you the flexibility to select what’s right for your therapeutic development.
Conquer the High-Stakes Climb to Successful Cell Line Development
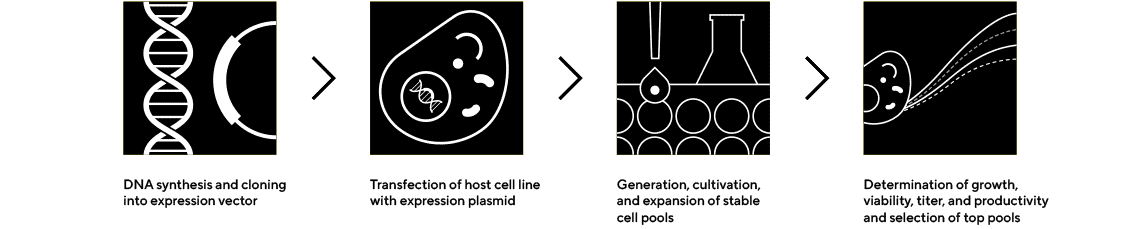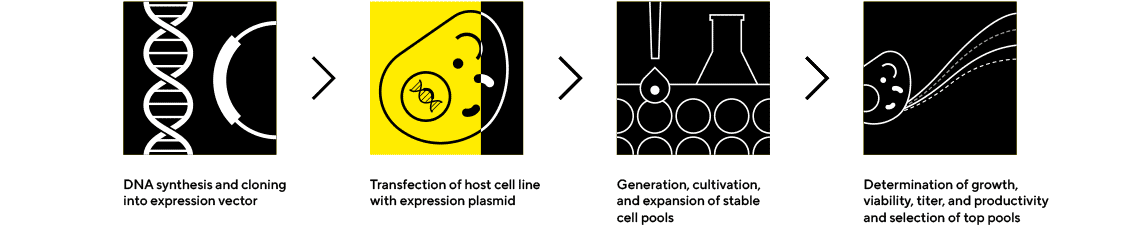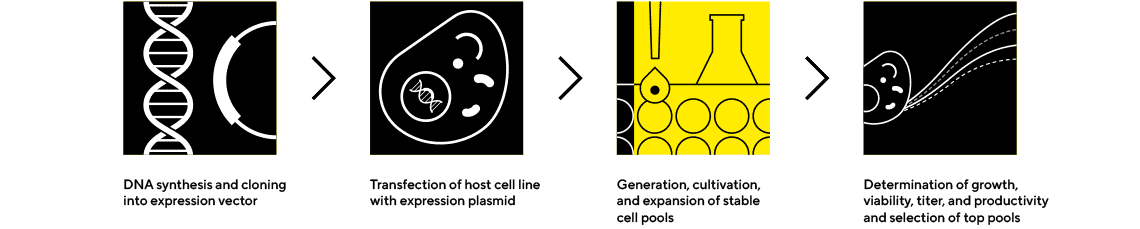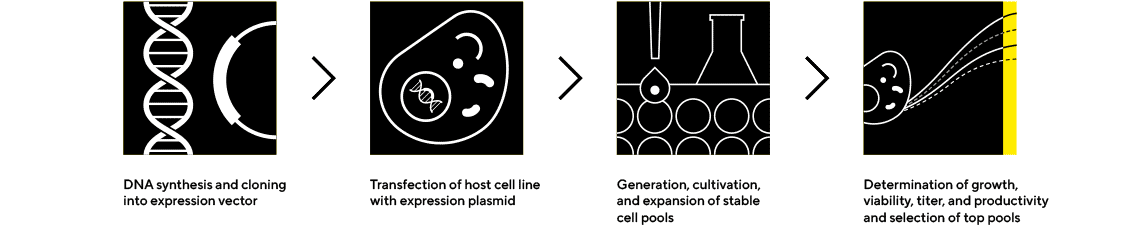
Cell line development is vital to biologics manufacturing, but the journey is time-consuming, expensive, and requires dedicated expertise.
Gene Cloning and Initial Clone Selection
Accurate, Rapid Cloning and Initial Clone Selection
We offer instruments and technologies to assist with transfection and clone selection. Our innovative solutions enable rapid selection of top pools after gene cloning and can help evaluate overall clone performance, from protein concentration to cell health and viability.
Cell Count and Viability
iQue® 3 High-Throughput Cytometry Platform
Use cell count and viability information to select the best pools. The iQue® 3 High-Throughput Cytometry Platform provides accuracy across a large linear range in 96- and 384-well plates and reproducible analysis of absolute cell count and viability data from a variety of non-adherent cell lines with a streamlined workflow, from cell labeling to analysis, with no cell dilution required.
- An integrated instrument, software, and reagent system for rapid, multiplexed analysis
- A unique patented sampling method that minimizes sample size requirements
- Deliver content-rich results in just minutes
Ready-to-Use, High-Performing Cell Line
Cell line development is a complex process, requiring consistency and reproducible results. Sartorius offers a ready-to-use solution with all of the necessary components for customers who prefer to develop cell lines and express their proteins of interest in their own facilities. The CLD Technology License is based on the industry-leading Cell Line Development Technology and uses CHO DG44 host cells. Because the technology license eliminates the need for further development or optimization, it significantly reduces development time and costs.
- Ready-to-use solution
- Well-performing cell line
- Reduced time and financial investment
Octet® Label-Free Detection
Easy Titer Determination of Active Analyte
Replace ELISA and HPLC with Octet® Label-Free Detection Systems for quantitation of antibodies and recombinant therapeutic proteins. Accurate and fast assays in real-time are easy to develop and transfer to QC and process development.
- Analyze a full plate (96-samples) of IgG titer in as little as two minutes
- Assay directly in crude and unpurified samples
- Optional automation for walkaway high-throughput analysis
Transfection Efficiency
Incucyte® Live Cell Analysis System
Monitor and quantify the efficiency and time course of gene transfection using green fluorescent protein (GFP) and red fluorescent protein (RFP) markers. Incucyte® Live Cell Analysis Systems use integrated image processing to identify wells containing emerging colonies.
CLD Services
Produce a High-Quality, Antibody-Secreting Clone Using a Proven Process
Sartorius’ cell line development services are targeted to large-scale production of biopharmaceuticals in mammalian cells, primarily Chinese hamster ovary (CHO) cells. The unique Cell Line Development service offers speed and flexibility, with a track record of performance and scalability.
- From DNA to fully characterized RCB in 9 weeks
- More than 240 projects successfully completed
- Save 3 months by omitting scalability studies
- Easy and direct scalability from small scale to 2000 L
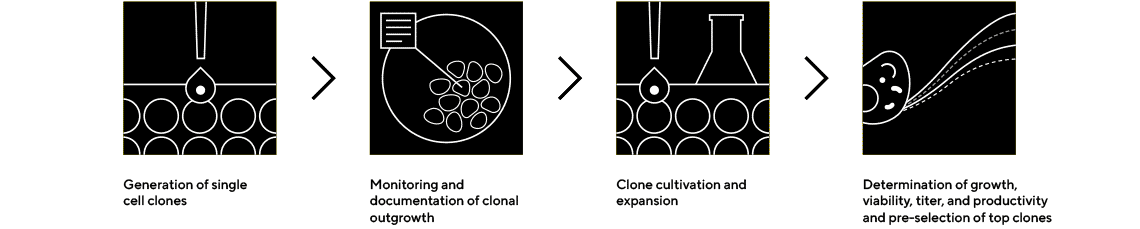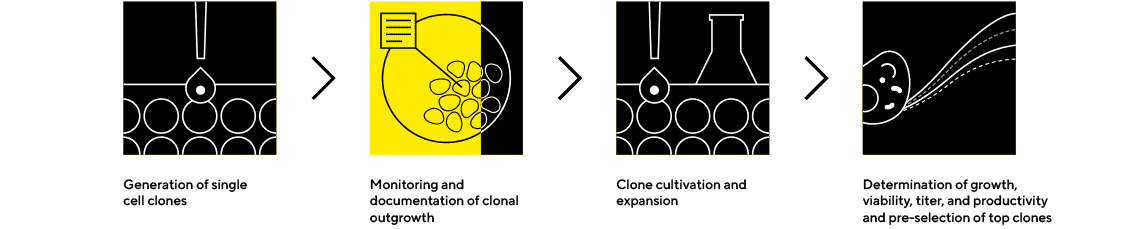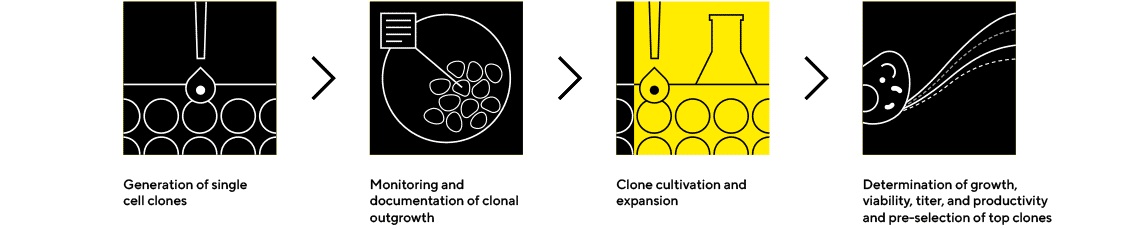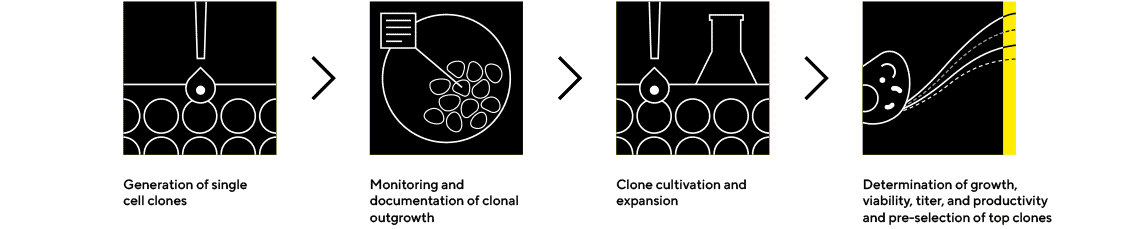
Single Cell Cloning
Single Cell Cloning with Integrated Monoclonality Verification
The CellCelector single cell and colony picking platform is used for high-throughput single cell cloning, allowing fast generation of clonal production cell lines. Cell lines are produced in one round while providing in-process, image-verified monoclonality proof. Integrated monoclonality and clone viability assessments - combined with high outgrowth rate after clone transfer to 96-well plates - the CellCelector is the leading alternative to limiting dilution or FACS single cell sorting techniques. High image quality ensures robust, automated, label-free single cell cloning.
- 100% selective clone recovery without cross contamination
- High outgrowth efficiency of transferred clones
- Reduced wait times for traditional growth and productivity measures
Cell Line Selection and Confirmatory Analytics
Expansion and Screening to Identify the Highest-Performing Clones
Early screening of product quality attributes in addition to quantity can facilitate the selection of clones to avoid late-stage failures. Ensure success by selecting the best clones based on quality and quantity using our instruments and package of ready-to-use assays and platform method.
Glycan Screening & Protein Quantitation
Octet® Glycosylation and Titer Determination Kits
Accelerate antibody discovery by combining titer data with sialic acid or mannose data for more informed decisions. Our glycosylation kits can screen and quantify proteins in crude and purified samples.
- No sample purification or digestion required
- Reduce preparation time up to 3 hours
- Screen for samples with desired glycosylation profiles
- Prevent late failures by moving higher-quality candidates downstream
Explore Octet® Glycosylation Kits
IgG Titer and Cell Health
iQue® Human IgG Titer and Viability Kits
Combine IgG quantification, cell counting and viability measurement in a single well without sample dilution. Quickly and efficiently learn critical productivity attributes for each clone:
- More insight for better clone selection
- More critical productivity attributes
- More clones screened in less time
Monoclonality
Incucyte® Live Cell Analysis System
Use whole-well, label-free imaging to automatically scan for clones and verify monoclonality. Integrated image processing identifies wells containing emerging colonies, allowing for the rapid identification of clones of interest.
Protein Quantitation
Octet® Label-Free Detection System
The Octet® BLI platform is an easy-to use, real-time biologics analytical tool that requires no labeling and enables rapid screening of critical quality attributes earlier in the decision-making process.
- Monitor titers before and after new conditions are introduced — e.g., supplements in cell growth media
- Measure directly in unpurified samples such as cell culture supernatants
- Process the sample plates further, thanks to non-destructive measurement
Quantitate Cell Count
Quantitate Cell Count and Viability Efficiently and Accurately
The CellCelector single cell and colony picking platform is used for high-throughput single cell cloning, allowing fast generation of clonal production cell lines. Cell lines are produced in one round while providing in-process, image-verified monoclonality proof. Integrated monoclonality and clone viability assessments - combined with high outgrowth rate after clone transfer to 96-well plates - the CellCelector is the leading alternative to limiting dilution or FACS single cell sorting techniques.
- Characterize single cells quickly and efficiently with integrated monoclonality and viability assessments
- No need for multiple cell pooling steps
- 100% selective clone recovery without cross-contamination
Cell Line Cultivation and Media Optimization
Quickly Produce Higher Protein Titers
Improved culture conditions and enhanced culture analysis give rise to optimized cell environments that maximize cell growth and metabolism. Sartorius offers a range of products and expertise to help you improve productivity and quality, enhance stability and reduce cost.
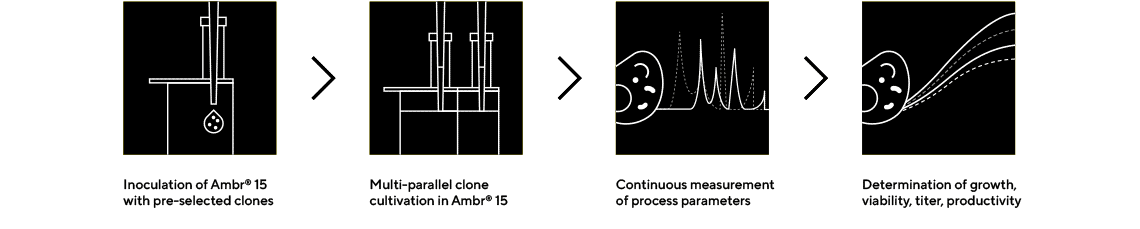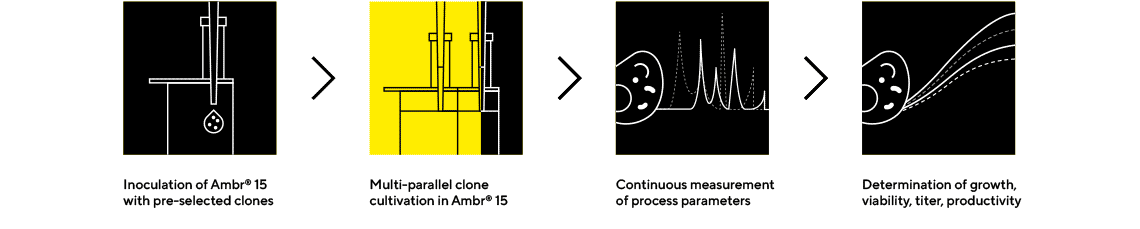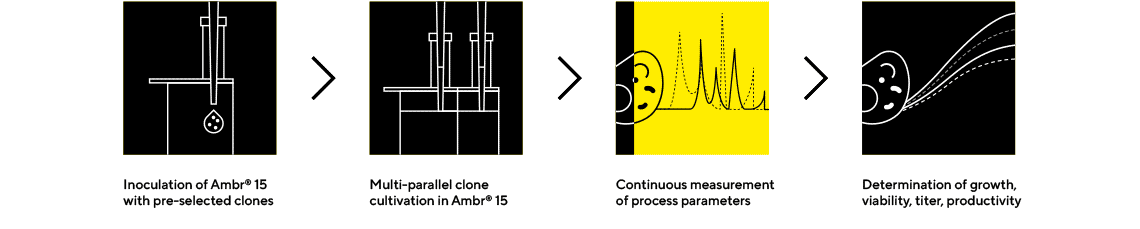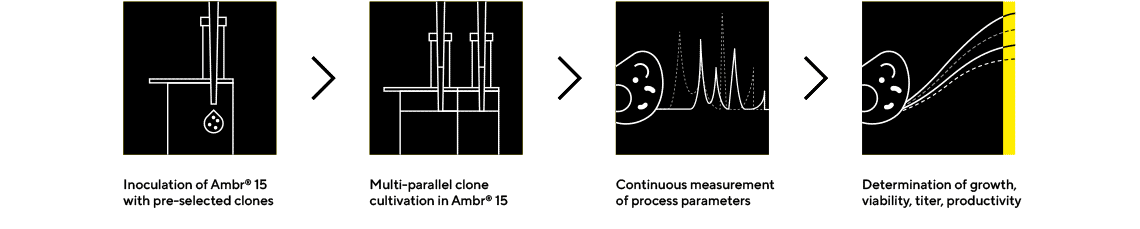
Quickly Produce Higher Protein Titers
Process parameters, cells and cell culture media interact in a challenging way to define the productivity and quality of the protein output. Sartorius ensures you get it right the first time, providing process-integrated media services to benefit your upstream process and reduce your time-to-market.
Explore Media Optimization
Multiparallel Cell Culture Evaluation
Ambr® 15 Cell Culture
The leading bioreactor system for cell line development and process optimization, Ambr® 15 allows parallel operation of up to 48 single-use microbioreactors (10-15 mL) controlled by an automated workstation. It increases productivity in cell line development with significant savings on materials and labor.
- Proven industry-standard microbioreactor for cell culture
- Better scale-down model than a shake flask
- High throughput and cost-effective
Protein Quantitation
Octet® Label-Free Detection System
The Octet® BLI platform is an easy-to use, real-time biologics analytical tool that requires no labeling and enables rapid screening of critical quality attributes earlier in the decision-making process.
- Monitor titers before and after new conditions are introduced — e.g., supplements in cell growth media
- Measure directly in unpurified samples such as cell culture supernatants
- Process the sample plates further, thanks to non-destructive measurement
IgG Titer and Cell Health
iQue® Human IgG Titer and Viability Kits
Combine IgG quantification, cell counting, and viability measurement in a single well without sample dilution. Quickly and efficiently learn critical productivity attributes for each clone:
- More insight for better clone selection
- More critical productivity attributes
- More clones screened in less time
Use DOE and MVDA to Characterize and Optimize Cell Culture Conditions
Umetrics® Suite helps you to quickly identify the optimal bioreactor and media conditions to achieve high protein titers.
MODDE® makes Design of Experiments (DOE) easy, allowing you to efficiently plan and analyze culture experiments.
SIMCA® lets you use Multivariate Data Analysis (MVDA) to understand correlations in multiple cell culture parameters as part of a Quality by Design (QbD) approach for scale-up.
- Reduce the number of experiments while maximizing information
- Quickly identify critical process parameters (CPPs)
- Increase process understanding
- Identify robust and optimal conditions (design space)
Cell Line Evaluation and Characterization
Quantify Protein of Interest and Screen Critical Quality Attributes
A suite of instruments and services evaluate selected clone(s) for contaminants, viruses, sterility, and protein production. A key aspect is defining Critical Quality Attributes (CQA) and monitoring these closely to ensure the characteristic attributes of the protein remain intact.
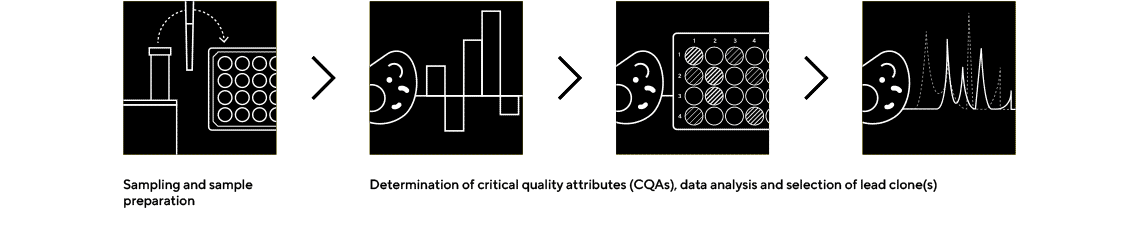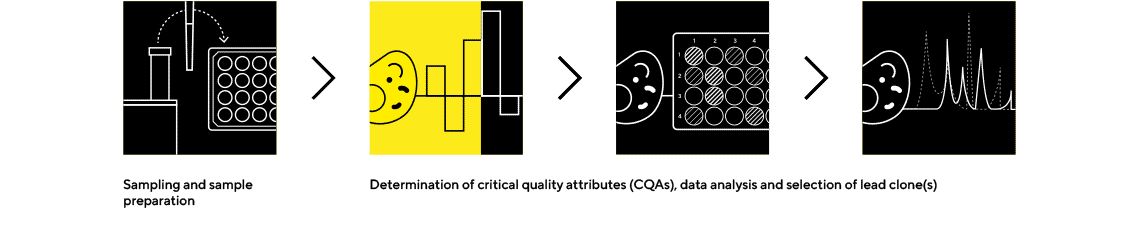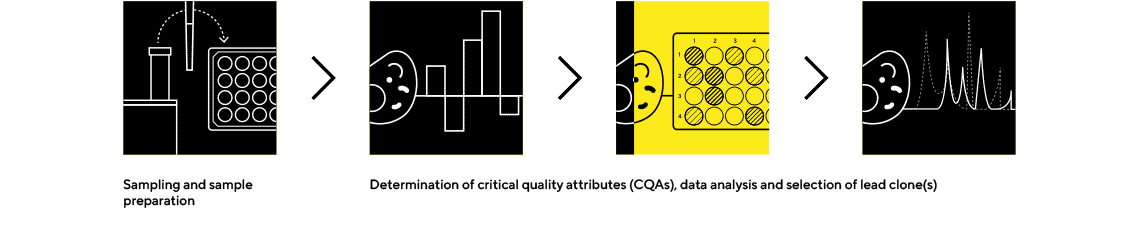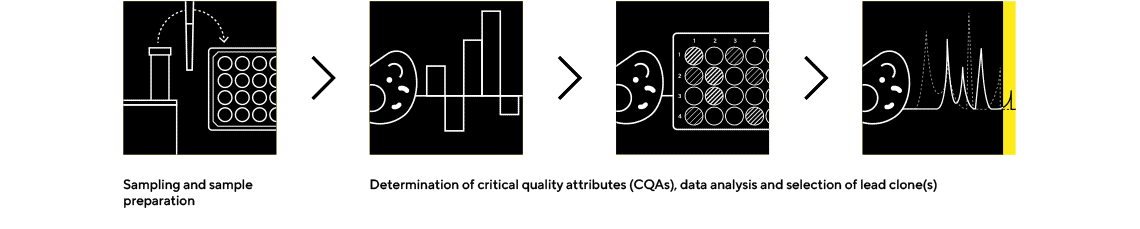
Octet® BLI Plattform
Rapidly Quantify the Protein of Interest and Screen Critical Quality Attributes
The Octet® BLI platform is an easy-to use, real-time biologics analytical tool that requires no labeling and enables rapid screening of critical quality attributes earlier in the decision-making process.
- Titer determination of active analyte
- Evaluate critical quality attributes (CQAs) such as glycosylation profiles
- Monitor product quality and stability during process development
IgG Titer & Cell Health
iQue® Human IgG Titer and Viability Kits
Combine IgG quantification, cell counting and viability measurement in a single well without sample dilution. Quickly and efficiently learn critical productivity attributes for each clone:
- More insight for better clone selection
- More critical productivity attributes
- More clones screened in less time
Cell Banking
Cell Banking Platform and Services to Create a Single Source for Manufacturing
Fully characterized, documented, homogenous master cell banks and working cell banks are critical to mitigate risk and ensure product safety and quality. Sartorius CHO cell banking platforms allows technology transfer of your own cell culture parameters without the need for engineering or pilot runs for an easy pivot to cGMP manufacturing.
Mammalian Cell Banks
Closed-System Cell Bank Manufacturing Platform
Our experienced team offers you the flexibility to do your cell bank manufacturing with us. Sartorius CHO cell banking platform allows technology transfer of your cell culture parameters into the platform without engineering or pilot runs.
- Dedicated cGMP facility
- Standard >500 vials, 12 x 106 viable cells/cryovial
- Closed and automated single-use
- Flexibility of transfer to any CMO
- Qualified Person (QP) batch release
- FILL-IT® automated filling: 500 vials in 20 minutes
cGMP Biosafety Testing
Cell Bank Biosafety Testing and Characterization Packages
Add ready-to-use testing schemes to existing drug development risk strategies or customize them. Sartorius QC testing plans meet mammalian cell bank release requirements and ICH Q5A regulations with GMP-validated assays. Sartorius has performed testing on over 200 CHO cell banks, so you can count on our experienced scientists to recommend the most suitable — and cost-effective — testing strategy to meet regulatory requirements.
- Expand QC workbench capacity and scientific expertise
- Accelerate time to market with rapid PCR or ELISA assays
- Gain access to testing experts
Discover Cell Line Characterization
Supporting Products/Solutions
CHO Media Sample Kits
Sample media for recombinant proteins and monoclonal antibodies (mAbs).
Request Your Sample
Related Resources
Explore Our Related Resources for Cell Line Development
Sartorius is a leading provider of cell line development solutions and services targeted to large-scale production of biopharmaceuticals in mammalian cells. Keep reading below to learn how we can help you on your journey to a master cell bank.