Monoclonal Antibody Discovery & Development
Monoclonal antibodies (mAbs) have already proven their effectiveness in treating a wide range of diseases. Their low toxicity and high specificity make them an attractive target for clinical research and investment. While oncology remains the leading therapeutic area for mAbs, their use has expanded to other diseases including neurodegenerative disorders, diabetes, and infectious diseases.
With hundreds of candidates in the pipeline, we anticipate the potential applications for mAbs to expand even further in the coming years.
In order for a successful monoclonal antibody (mAb), discovery and development program to address difficult target classes, you need innovative technologies that allow rapid identification and characterization of candidate molecules to identify those with superior target reactivity and to further optimize their functionality and developability.
Our innovative solutions for discovery and development of antibody therapeutics streamline the workflow and increase productivity.
Accelerate your antibody development and still have peace of mind.
Ensure confidence in your monoclonal antibody discovery and development program:
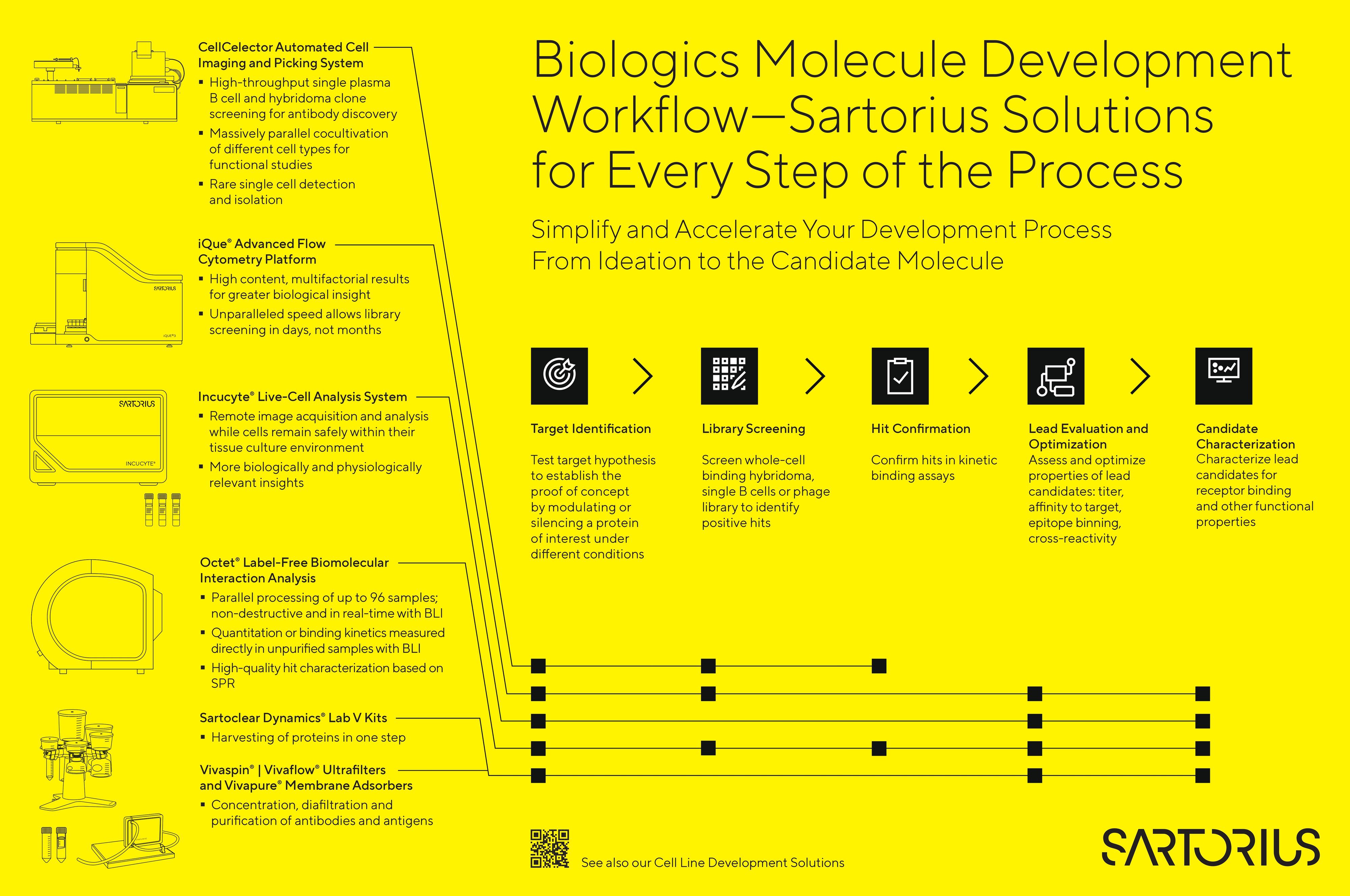
Product Highlights for Antibody Therapeutics Discovery & Development
Cell Analysis Platforms
CellCelector Automated Cell Selection and Retrieval Platform
Fully automated image-based screening and isolation of clones and single cells for antibody discovery. Single cell analysis with the CellCelector allows the detection of rare antibodies with unique properties which are hard to find under conventional screening methods.
- High-Throughput Identification of Monoclonal High Producer Clones
- Automated Single Plasma-B-Cell Secretion Screening and Recovery
- Selection of High-Producer Hybridoma and Chinese Hamster Ovary (CHO) Cells
Incucyte® Live-Cell Analysis System
Enables real-time, live-cell imaging and analysis directly inside your incubator. Measure kinetic effects of your antibody treatment on target cells without ever having to remove cells from the incubator.
- Use with non-perturbing Incucyte® reagents to detect dynamic biological changes
- Measure kinetic effects of your antibody treatment on target cells
- Accommodates multiple users and applications in parallel
iQue® 5 High-Throughput Screening (HTS) Cytometry Platform
The new iQue® 5 is designed for speed, simplicity and scalability. Perform simultaneous phenotype and functional analysis of secreted cytokines. Drive progress in automated antibody discovery and screening, T-cell engineering and immuno-oncology.
Don’t miss the chance to experience:
- Next-level performance
- Effortless operation
- Powerful and intuitive software
- Simplified, extended runs
Protein Analysis Platforms
Octet® Label-Free Biolayer Interferometry (BLI) Detection Systems
Octet® systems are used for affinity ranking, epitope binning of small to large antibody matrices, Fc-receptor binding, cross-reactivity testing of other species, glycosylation screening, characterization of antibody-antigen binding kinetics and affinity, and titer analysis for protein expression, all in one high throughput, easy-to-use system.
- Measure molecular interactions in real-time without the need for detection reagents
- Advanced, fast, and direct detection of specific biomolecules
- Rapidly determine critical quality attributes (CQAs) and perform off-rate analysis to select optimal clones using both purified and unpurified samples
Octet® SF3 Surface Plasmon Resonance (SPR)
With exceptional sensitivity for both small and large molecules, the Octet® SF3 allows users to generate high-quality kinetics and affinity data in a fraction of the time compared to standard multi-cycle kinetics.
- Complete kinetics and affinity for up to 768 samples in a single unattended assay
- Eliminate the need to prepare multiple dilution series using OneStep® Injection Technology
- Determine full kinetics and affinity in the presence of multiple competitors