The Progress and Promise of iPSC-derived Therapies
Induced pluripotent stem cells (iPSCs) are a type of stem cell that can be generated directly from adult cells. The history and importance of iPSCs are significant in the field of regenerative medicine, biology, and the potential treatment of various diseases.
The Progress of iPSCs:
- Discovery: The concept of iPSCs was first introduced by Shinya Yamanaka and his team at Kyoto University in Japan in 2006. They demonstrated that the introduction of four specific genes encoding transcription factors (Oct3/4, Sox2, Klf4, and c-Myc) could reprogram adult mouse fibroblasts to become pluripotent stem cells.
- Human iPSCs: Following the success with mouse cells, in 2007, Yamanaka's team and a group led by James Thomson at the University of Wisconsin-Madison independently developed human iPSCs. This breakthrough opened up new possibilities for stem cell research and medical applications in humans.
- Nobel Prize: In recognition of his groundbreaking work, Shinya Yamanaka was awarded the Nobel Prize in Physiology or Medicine in 2012, which he shared with Sir John Gurdon, who had earlier work on nuclear transplantation in frogs that laid the foundation for the field of cellular reprogramming.
The Promise of iPSCs:
- Disease Modeling: iPSCs can be derived from patients with specific diseases, allowing researchers to create cell models of these diseases in a laboratory setting. This is particularly valuable for diseases that are difficult to study in vivo, such as neurological disorders.
- Drug Discovery and Testing: iPSCs can be used to test the efficacy and safety of new drugs. Since these cells can be generated from individuals with particular genetic backgrounds, they can also help in understanding the genetic factors that influence drug responses.
- Regenerative Medicine: iPSCs hold great promise for regenerative medicine because they can potentially be used to generate healthy cells, tissues, or organs for transplantation, which could be used to treat various conditions, including heart disease, diabetes, and spinal cord injuries.
- Personalized Medicine: iPSC technology could lead to personalized medicine, where cells derived from a patient's own body are used to treat their disease, reducing the risk of immune rejection.
- Genetic Correction: iPSCs can be used in combination with gene editing technologies, such as CRISPR/Cas9, to correct genetic defects in patient-derived cells, which can then potentially be used in therapeutic applications.
The development of iPSCs has revolutionized the field of stem cell research and has opened up new avenues for understanding human development, disease mechanisms, and the potential for cell-based therapies. However, there are still challenges to be addressed, such as ensuring the safety of iPSC-derived cells for clinical use, understanding the long-term effects of reprogramming, and developing efficient differentiation protocols. Despite these challenges, iPSCs remain a highly promising area of research with the potential to significantly impact medicine and human health.
The Progress and Promise of iPSCs
Check out this video to learn about the history and application of iPSCs as well as the critical steps in an iPSC R&D workflow.
The Top Things You Will Learn About
- History of iPSCs
- Benefits of using iPSCs in cell therapy research
- Steps in an iPSC R&D workflow
- Challenges associated with an iPSC-derived therapy workflow
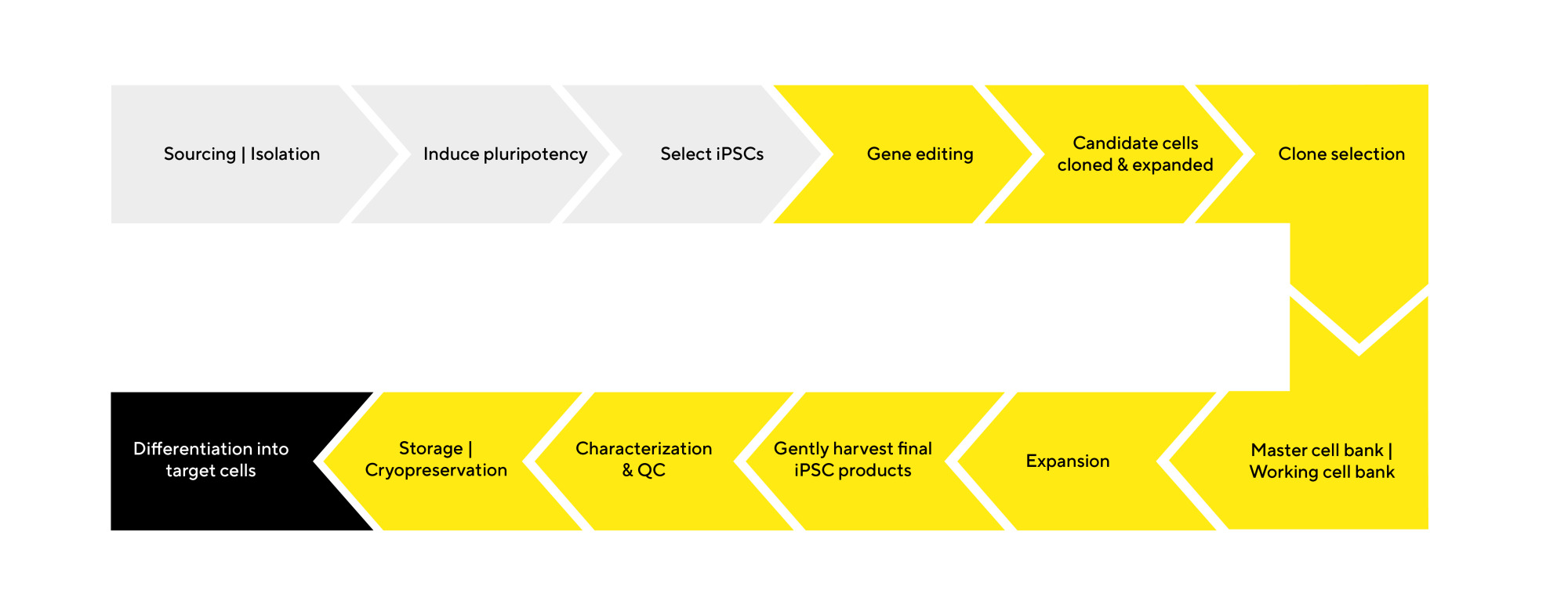
iPSC Therapy Research & Development Workflow
Frequently Asked Questions
iPSCs are created by introducing a set of specific genes, often called reprogramming factors, into adult cells. These factors reprogram the cells' DNA, turning them back into cells with pluripotent capabilities.
The main difference is their origin. Embryonic stem cells (ESCs) are derived from the inner cell mass of a blastocyst, an early-stage preimplantation embryo, while iPSCs are derived from adult somatic cells that have been genetically reprogrammed. Functionally, both cell types are similar in their pluripotent capabilities.
iPSCs are important because they offer a source of pluripotent cells that can be generated without the ethical concerns associated with the use of embryonic stem cells. They also allow for the creation of patient-specific cells, which can be used for personalized medicine, disease modeling, and drug testing.
iPSCs are still largely in the research and clinical trial phases for most applications. There have been some early clinical trials using iPSC-derived cells for conditions such as macular degeneration, Parkinson's disease, and heart disease, but widespread clinical use is still in the future.
Adult stem cells, such as hematopoietic stem cells found in bone marrow, are limited in the types of cells they can become. iPSCs, on the other hand, are pluripotent, which means they have the potential to differentiate into nearly any cell type in the body.
Yes, iPSCs can be generated from patients with genetic diseases, providing a powerful tool to study these diseases in the laboratory. Researchers can observe how the disease develops and progresses at the cellular level, which can lead to new insights and treatments.
The future of iPSC research is focused on improving the safety and efficiency of cell reprogramming, developing better methods for differentiating iPSCs into specific cell types, and advancing the use of iPSCs in clinical therapies. Additionally, combining iPSC technology with gene editing tools like CRISPR could lead to breakthroughs in treating genetic disorders.