Complete AAV Testing Service From Discovery to Commercialization
Adeno-associated virus (AAV) offers advantages in gene delivery like long-term gene expression, non-pathogenicity, and very low immunogenicity.
But AAV manufacturing comes with challenges. Regulatory requirements are complex and evolving, aggregation occurs across serotypes, and products can form full, empty, and partially filled capsids. AAV is also non-replicating, making process and product characterization extremely challenging.
To support your AAV manufacturing, Sartorius offers AAV testing services from R&D to regulatory approvals – with guaranteed testing slots and expertise from our experienced and dedicated team.
Save time and costs with our ready-to-use QC testing plans and pre-validated cGMP assays.
Use specialized high-quality analytics and product release assays throughout development.
Leverage years of experience from our dedicated team of experts in each step in your process.
Approaches to AAV Characterization
Navigate Regulatory Requirements With Ease
The regulatory landscape is complex and evolving. Authorities constantly release new AAV guidance, and even small missteps can make approvals more time-consuming and costly.
We work with you to develop, establish, and validate your AAV characterization for metrics like safety, identity, purity, and potency. Our team has experience navigating approvals for the FDA and EMA/CAT, in addition to guidance from Korea, Japan, and China.
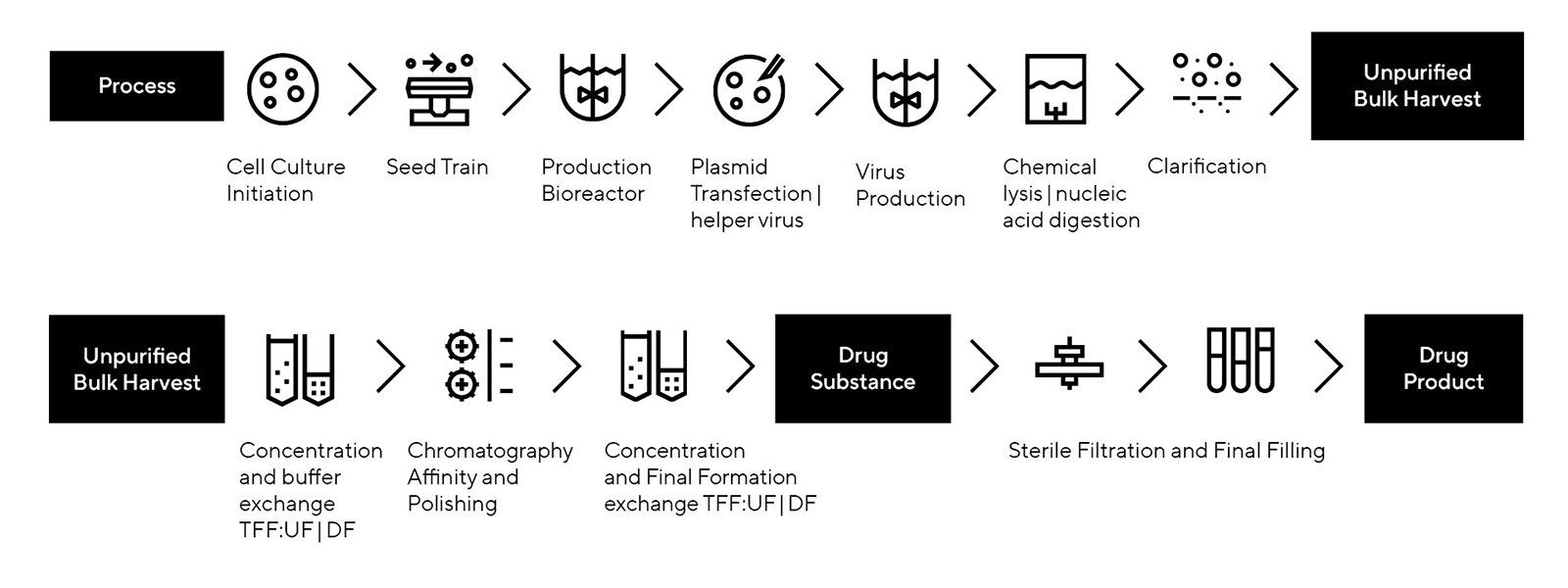
Methods to Test the Following Attributes
Confirm the unique identity of your product and distinguish it from any other constituents of the product or process .
- Capsid identity
- Gene of interest
- Whole genome sequencing
Demonstrate that your finished product minimizes extraneous matter.
- Full: Empty capsid ratio
- Residual HCP, DNA, BSA, benzonase, ligands, and plasmids
- Transfection agents and surfactants
- Aggregation
Define the therapeutic activity of your drug.
- Infectivity
- Genomic titer
- Viral particle titer
Ensure your product is free from adventitious agents including viral and microbial agents.
- Sterility and mycoplasma
- Endotoxin
- Adventitious virus
- Replication competence: rcAAV
- Species-specific virus
Simplify Product Characterization in Early Development
In early development, save time on basic product characterization by outsourcing testing methods. Our selection and expertise enable you to focus on process development, so you can increase final yield and move to the next development stage sooner.
Streamline the Transition From Early Stage to Clinical Trials
When an AAV drug candidate is considered ready for pre-clinical studies, manufacturers need to perform a complete characterization by evaluating product- and process-related impurities.
Support your AAV product’s entry into clinical trials with our broader product characterization, including general product quality and physicochemical aspects. Our testing methods are designed to help you meet regulatory requirements along with generic qualification of assays.
Speed up Late-Stage Development and Commercialization
To enter later clinical trials and navigate commercialization, you need complete product characterization that meets regulatory requirements, as well as testing methods validated for your specific AAV product and phase.
Leverage our pre-qualified testing methods and off-the-shelf assays to make turnaround times fast. If you need product-specific optimization, our team has developed and qualified assays for the most used AAV serotypes.
Testing Methods & Regulatory Guidelines
Read the table below for an overview of testing methods as mandated by regulatory guidelines, as well as the testing to perform at each development stage.
The measurement of transfection reagent present in an AAV sample is a requirement of regulators to show that a manufacturer has a well-controlled process for minimising this impurity as it can be potentially toxic to cells.
- PEIpro® is a PEI based transfection reagent for scalable viral vector production (both adherent and suspension systems)
- FectoVIR®-AAV is a novel generation of synthetic transfection reagent specifically developed for industrial scale production of recombinant AAV (rAAV) in both suspension and adherent HEK-293 derivative cell systems
Sartorius have developed specific GMP UHPLC methods for detection of residual PEIpro® and FectoVIR®-AAV integrated within the wider AAV testing portfolio.
Our Product Portfolio
More Cell & Gene Therapy Solutions From Sartorius
We share your mission to develop life-changing cell and gene therapies. Accelerate commercialization with end-to-end solutions that deliver reproducible performance and scalability with a simplified path to regulatory approvals.
FAQs - AAV Services
Yes, we offer GMP-compliant methods to help you meet regulations at clinical and commercial phases, as well as pre-clinical appropriate non-GMP methods.
Our AAV testing adheres to guidance from the FDA and the EMA/CAT, as well as guidance from regulatory authorities in Korea, Japan, and China.
In January 2020, the FDA issued Chemistry, Manufacturing, and Control Information for Human Gene Therapy Investigational New Drug Applications, Guidance for Industry (FDA CBER January 2020), while Europe still applies Guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products (EMA/ CAT/80183/2014).
Each guidance document requires that all drug products are assured for safety, identity, purity, and potency.
Yes, we do. Please contact us if you’re interested in a method that isn’t on this page. We have more than 15 years of experience in developing and providing methods for our customers.
Please contact us to discuss testing for other viral vectors.
We have dedicated testing slots available for booking AAV testing in addition to our existing testing and cell banking services. We offer worldwide coverage and weekly updates on lead times.