MES Apps Built for Speed and Seamless Integration
Reimagined for Single-Use Bioprocessing
Biobrain® Operate powered by Tulip is the first manufacturing execution system (MES) built specifically for Sartorius bioprocess equipment and single-use bioprocessing. Built on Tulip´s cloud-based, no-code platform, it digitizes standard operating procedures (SOPs), electronic batch records (eBRs), and other critical bioprocessing workflows to:
- Reduce human errors
- Streamline QA reviews
- Accelerate operator onboarding
- Ensure consistent, paper-free execution
- Deploy up to 66% faster than traditional MES
Replace paper with digital SOPs and batch records that streamline execution, enable real-time review, and streamline release.
Minimize human error in single-use bioprocessing with guided digital workflows, cutting costly deviations and safeguarding quality.
Shorten release from weeks to days. Digital batch records enable real-time, reduced rework, and faster approvals.
The embedded digital guidance standardizes learning shortens onboarding and equips new operator work with confidence.
Enable future-ready operations with no-code digital workflows that adapt fast eliminate IT backlog.
Smarter SOPs for Biostat® STR
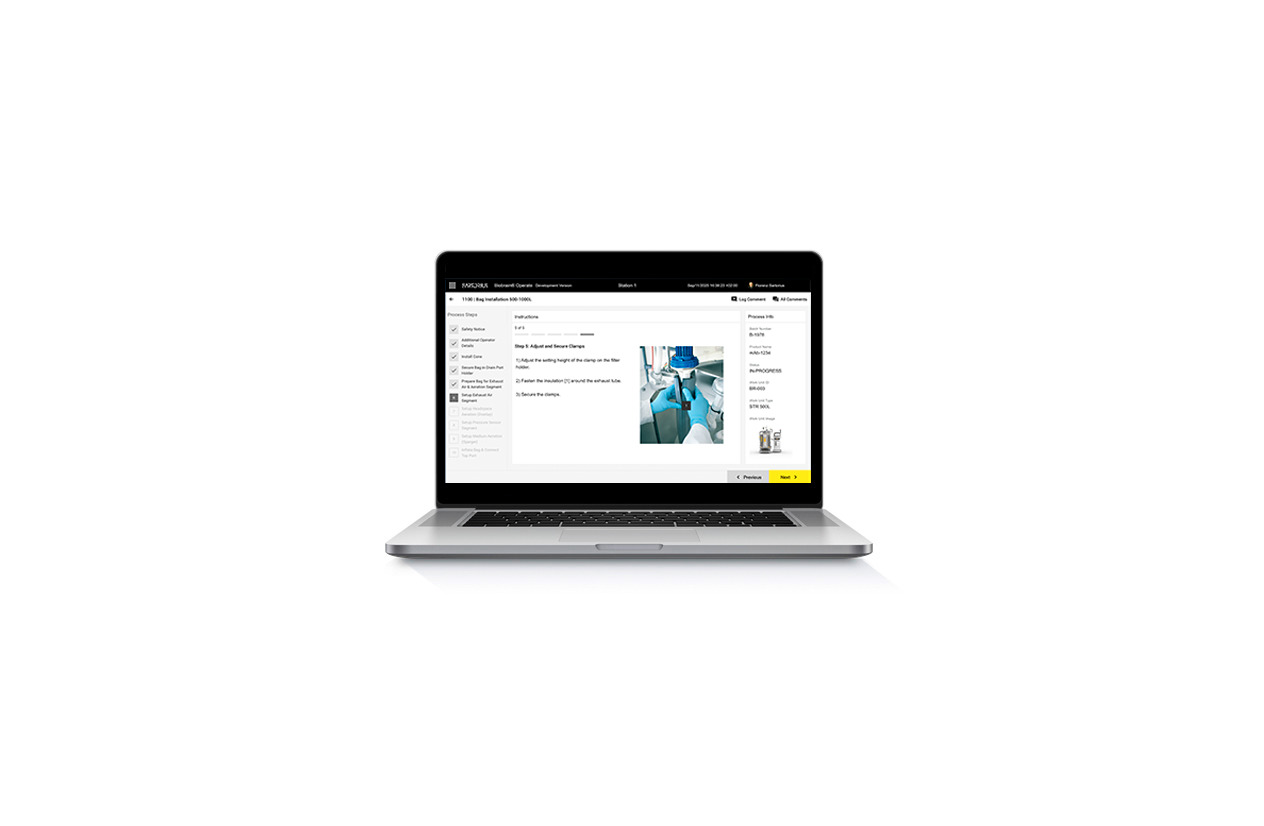
Bring your Biostat® STR processes into Biobrain® Operate with interactive eSOPs. Our step-by-step guidance, built-in checks, and media-rich instructions help operators execute consistently, reduce errors, and accelerate training — all while moving toward paperless bioprocessing.
A Digital Toolbox for the Cleanroom
Biobrain® Operate is a digital toolbox built for single-use bioprocessing. Instead of a heavy, one-size-fits-all system, it delivers focused apps for operators, QA, and facility staff — from MBRs to eSOPs, batch tracking, equipment management, and dashboards. We offer practical, adaptable, and ready for the cleanroom solutions.
Digital MBR Authoring
Replace Static Templates With Dynamic Records
Design, update, and manage Master Batch Records (MBRs) digitally. Transform static templates into dynamic assets that flow into SOPs, eBRs, and execution — creating one connected MES ecosystem.
- Streamlined authoring – build complex processes with clarity and speed
- Seamless execution – MBRs flow directly into SOPs and eBRs, & execution
- Dynamic updates – easily revise and version MBRs as processes evolve
- Faster approvals – streamlined templates speed up approvals
Reduce operator errors and speed execution
Replace paper standard operating procedures (SOPs) with fully electronic, connected SOPs. Reduce transcription errors, missing entries, and delays from manual sign-offs. Every step is time-stamped, traceable, and audit-ready.
- Operator Friendly – Clear, visual, and easy to follow
- Error Mitigation – Built in checks to reduce mistakes
- Instant QA Connection – Escalations trigger real-time quality input
- Always Current – Operators follow the latest version
Create secure, traceable, and audit-ready documentation
Electronic batch records (eBRs) accelerate batch review and release by providing a fully digital, traceable record of production execution. All data is captured in real-time, securely archived, and fully compliant – giving QA teams the ability to focus on exceptions, not every line item.
- Reduced Review Burden – QA effort minimized with exception-based-review
- Accelerated Release – Faster approvals and shorter review cycles
- Not Buried in Binders - Digital records are searchable and accessible 24/7
- Data Integrity – Secure, traceable, audit-ready records
Track usage, maintenance, cleaning, and calibration centrally
Electronic logbooks (eLogbooks) provide a complete history of equipment usage and maintenance. Every entry is captured electronically, creating a reliable record of readiness across assets. With centralized visibility, teams can confirm status in real-time, eliminating the burden of chasing paper logbooks across the plant.
- Lifecycle Tracking - Full history of use, service, and maintenance
- Centralized Visibility – One source of truth across all assets
- Secure Records – eLogbooks replace scattered binders
- Reduced Downtime – No delays waiting for logbook checks
Gain transparency across sites, teams, and operations
Dashboards turn raw data into actionable insights. Live charts and KPIS show batch, equipment, deviation, and reporting trends. QA and operations con spot bottlenecks, respond faster, and benchmark performance – all without digging through static reports or spreadsheets.
- Operational Visibility – Monitor real-time performance and readiness
- Deviation Trends – Track quality issues and follow-ups over time
- Bottleneck Alerts – Identify process hold-ups before they cost time
- Custom Views – Build dashboard tailors to your workflows with no-code tools
Powered by Tulip’s No-Code Platform
Seamless integration, no-code power
Biobrain® Operate powered by Tulip, gives you the flexibility of a no-code platform with the rigor biopharma demands. Build apps with drag-and-drop simplicity, connect to equipment and enterprise systems (LIMS, ERP, QMS), and stay compliant with full audit trails, governance, and role-based control.
- Fully configurable
- Cloud-based
- GxP-ready
FAQs
MES streamlines bioprocessing by digitizing SOPs, batch records, and QA workflows, improving efficiency and compliance.
eSOPs replace paper procedures with step-by-step guidance that ensures consistency, reduces human error, and speeds onboarding.
Digital guidance provides real-time instructions, checks, and validations that help operators work right-first-time and reduce costly deviations.
eBRs capture operator actions and process data digitally, enabling faster QA review, audit readiness, and paperless compliance.
By enabling review-by-exception and real-time QA checks, electronic batch records cut release cycles from weeks to days.
Paperless bioprocessing replaces manual records with secure digital data, improving integrity and traceability. It reduces errors, streamlines workflows, and enables compliant, efficient operations across sites — while also cutting paper use and supporting sustainability goals.
A no-code platform lets process teams adapt workflows quickly without IT support, speeding deployment and scaling across facilities.
Legacy MES is costly and rigid. Lean, modular systems like Biobrain® Operate powered by Tulip bring faster adoption, flexibility, and lower risk.