N-1 Perfusion Equals Lower COGS, Higher Titer
N-1 Perfusion High Inoculum Fed-Batch Is a Logical First Step Toward Intensification
With increasing development cost (>30%) and reduction in sales (<50%) biomanufacturers need to increase productivity of their facility to reduce overall COGS to stay competitive. Intensifying their seed train, especially the N-1 step, is one of the most adapted and logical change that the manufacturers can implement.
バイオ医薬品分野の課題に対応する柔軟なプロセス強化
本ウェビナーでは、ザルトリウスの専門家が最新 のプロセスインフォメーション技術をご紹介いたします 。これには、Biobrain®自動化プラットフォームによって制御されるPAT機能を搭載した 進化した Biostat®バイオリアクターも 含まれます。
N-1パーフュージョンにより、抗体価が2倍に上昇し、全工程の持続時間を短縮し、スループットを20~50%向上させることが可能です。
スループット向上により、追加の培地要件がコストや設備の設置に与える影響は軽減されます。
N-1は、メインのバイオリアクターにおけるフェドバッチ戦略を変更することなく、既存のフェドバッチ設備への容易な改造を実現します。
N-1パーフュージョンは、より高いスループットと生産性の向上により、従来のフェドバッチ方式と比較して、グラム当たりのコストを30~50%削減します。
強化された種培養の選択肢
N-1 for High-Performing High Inoculum
In the N-1 perfusion high inoculum fed-batch process, the production bioreactor is seeded at a higher density, accelerating cell growth. Biomanufacturers can leverage this benefit in two ways:
- Achieve the same titer as standard fed batch in 40% fewer days, increasing throughput
- Deliver almost double the titer in the same time as standard fed batch operations
Sartorius is the only company offering N-1 seed train intensification options for both rocking motion and stirred tank bioreactors.
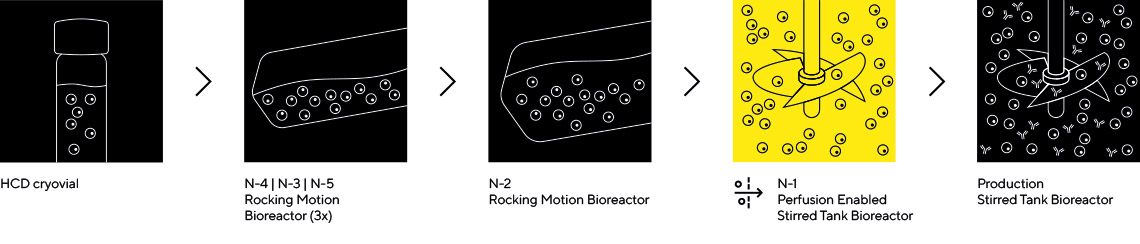
A Cost-Effective Upstream Intensification Option
Implementing rocking motion bioreactor, with integrated perfusion membrane, in the N-1 seed train step is the most cost-effective opportunity for seed train intensification for high inoculum fed-batch process. Using the RM bioreactor manufacturers can
- Reduce both capital and consumable investment by >30%
- Achieve high cell densities at lower volumes to inoculate Production Bioreactor
- Increase productivity and overall facility throughput by 30-50% thus decreasing overall COGS by up to 50%
Intensification Made Simpler with Perfusion-Ready Biostat STR®
Sartorius Biostat STR® bioreactors are available with integrated XCell ATF® technology, enabling an easy switch form batch to perfusion processes in any stage of the seed train. Implementing intensification or perfusion in the seed train achieves a higher N-1 cell density that, in turn, enables a higher inoculation cell density of the N bioreactor. This provides considerable benefits:
- Easy retrofit in the current fed-batch facility
- Increased titer (up to 2 times)
- Reduced COGS (up to 50%)
- Increased overall facility throughput >50%
Media Management
Ensuring Media Quality and Stability Is Critical for Intensification
Running the N-1 seed train step in perfusion mode requires a larger amount of cell culture medium than in standard fed-batch. Ensuring consistent cell culture performance in intensified processes is dependent on the media design, quality, and stability. Consumables and equipment to handle these volumes are equally essential to support a smooth process flow. A reliable filter to avoid contamination and remove mycoplasma is also critical for successful intensified processes.
The Flexsafe® Pro Mixer Saves Time Without Compromising Control
Flexsafe® Pro Mixer is an efficient, single-use mixer for 50 L up to 3000 L. It provides the high levels of control that are essential for cGMP biomanufacturing.
- Saves time with fast powder dissolution
- Preserves quality of shear sensitive fluids through low shear mixing and high torque
- Designed for rapid set-up and efficient changeover (in less than 10 minutes) reducing risk of damage and loss from product mishandling
- Includes full material characterization and safety evaluation
Single-Use Bags
2D Single-Use Bags
Storage to Support the Media Volume Required for N-1 Perfusion
N-1 perfusion requires more cell culture medium, so consumables and equipment to handle these volumes are essential for a smooth process flow. Sartorius Flexsafe® 2D Single-Use Bags ensure the stability and integrity of the medium, allowing excellent, reproducible cell growth. The superior strength of the PE film reduces risk of damage, while its flexibility enables consistent performance and easy handling.
3Dシングルユースバッグ
パレットタンク用自動展開・充填式3Dシングルユースバッグ
液体培地は、必要な時にいつでも使用できるよう、事前に調製し安全に保管しておく必要があります。N-1パーフュージョン培養における多量の細胞培養用培地需要に対応し、円滑なプロセスフローをサポートするためには、大規模な液体貯蔵が求められることが多々あります。 パレットタンク内での保管アプリケーション向けに設計されたFlexsafe® 3Dシングルユースバッグは、操作が容易で、優れた再現性のある細胞増殖を可能にします。自動展開アクセサリーにより、オペレーターの操作なしにバッグへの充填が可能です。これらの即使用可能なバッグは、Sartorius Biostat STR®バイオリアクターとの「プラグアンドプレイ」対応を備えています。
Ensuring Quality and Safety of Cell Culture Medium
Companies are looking more and more for virus removal filters for media for risk mitigation purposes. Virosart® Media filters, provide high virus clearance safety and show superior capacities for media used for mAbs and recombinant proteins.
Cost Efficient Media Filtration with High Mycoplasma Retention
Sartopore® 2 XLM sterile filters are used for cost-efficient media filtration with outstanding high throughput. They feature a 30% larger surface area and optimized pre-filter layers which results in a higher flow rate and higher throughput. The Sartopore® 2 XLM is an advanced 0.1 µm rated filter with high mycoplasma retention.
バイオシミラー製造メーカーにおけるN-1パーフュージョンプロセスの導入
本ウェビナーでは、バイオシミラー製品の主要メーカーであるインタス・ファーマシューティカル社が、強化製造プロセスへの有効な入り口としてシードトレイン強化をどのように対象としたかをご紹介します。
- 製造シナリオにおける種培養強化の価値をご確認ください。
- 種培養の集約化を実現するための様々なアプローチについて学びましょう。
Process Data Analytics
Assurance through Multivariate Data Analytics
Customers rely on enhanced approaches to data analytics for process intensification. This is due to the increased complexity of processes, operations, and data, as well as the total cost of risk. Multivariate Data Analysis (MVDA) extracts meaningful information from large data sets. It enables scale-up and batch-to-batch comparison investigations to improve the quality, safety, and efficacy of a drug product.
SIMCA®はプロセスの健全性と製造の成功を保証します
SIMCA®は、大量のデータから実用的な情報を得るための進化したデータ分析ソリューションであり、一貫したプロセスと製品品質を確保します。SIMCA®は、投入される原材料を分析し、それらが細胞の増殖、生存率、生産性、品質にどのように影響するかを判断します。これはN-1パーフュージョンにおいて極めて重要です。この堅牢な多変量解析エンジンは、バッチ間の品質を比較し、あらゆる変動の原因を特定します。直感的なインターフェイスと分かりやすいインタラクティブな可視化を通じてアクセス可能です。
SIMCA® -onlineは、リアルタイムモニターと制御を統合します。
多変量リアルタイムモニターは、特に強化されたプロセスにおいて重要です。SIMCA®がプロセスの基準となるデジタル指紋を作成する一方、SIMCA®-onlineはリアルタイム測定値を用いてこの指紋に基づきオペレーションを評価します。つまり、現在のバッチ指紋が基準と一致しているかどうかを検証するのです。基準からの逸脱が確認された場合、プロセスを即座に調整することが可能です。この自動化された是正措置により、品質の一貫性が保たれ、効率が最大化され、コスト削減が実現します。