Concentrated Fed-Batch for Increased Productivity and Flexibility
Intensification With Flexibility for Higher Facility Throughput Requirement
Concentrated fed-batch (CFB) is the upstream process intensification that can have the greatest impact on productivity compared to standard fed-batch, producing up to four times the titer and 3-4 times higher throughput. Concentrated fed-batch is the ideal option for converting stainless-steel to single-use operations and for blockbuster drugs. Considering the increasing trend towards throughput of less than 500 kg per year, concentrated fed-batch offers the flexibility to have a multiproduct high throughput facility to satisfy productions of multiple drugs within the same single-use facility.
連続培養法(CFB)は、生産用バイオリアクター内での生細胞密度を高めることが可能であり、その結果、フェドバッチ法と比較して製品収量が最大4倍増加します。
CFBはスループットを最大3倍まで向上させ、ハイスループット施設(年間500kg以上)が市場の需要に応えるために必要な柔軟性を実現します。
設置面積が小さいため、シングルユースの施設はより持続可能であり、適応性と柔軟性に優れています。
Intensified Seed Train and Production Options
高密度フェドバッチによる高密度インパクト
製造が最大4倍向上、少ない容量や異なる製造方法においても
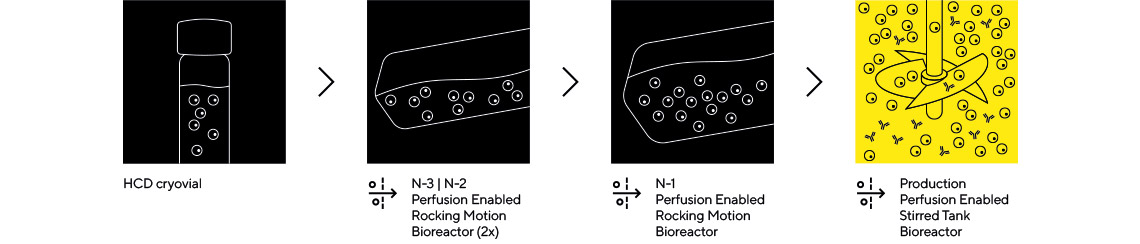
高密度フェドバッチ(CFB)は、種培養におけるN-1強化と組み合わせ、生産用バイオリアクターを濃縮モードで運転することにより、生産性を4~5倍向上させます。製品と細胞はバイオリアクターにフィードバックされ、濃縮されることで、最終ハーベストまで高い細胞密度と力価を達成します。最終ハーベストはバッチモードで行われます。このプロセスにおいて使用済み培地を除去するには、限外ろ過モードで使用される交互タンジェンシャルフローフィルターが極めて重要です。標準的なフェドバッチ法と比較して最大4倍の力価と3~4倍のスループットを実現するCFBは、多製品対応のシングルユース施設や高薬品需要施設にとって魅力的な選択肢です。
特許取得済み 揺動型バッグ(内蔵型パーフュージョンメンブレン付き)
揺動型パーフュージョンシステムは、パーフュージョン膜を内蔵した揺動型シングルユースバッグで構成される最適なソリューションです。独自の設計により、別途の細胞分離装置が不要となります。N-1種培養工程において揺動型パーフュージョンバイオリアクターを採用することは、強化されたアップストリーム種培養を確立する費用対効果の高い方法です。これにより生産性が向上し、種培養工程数の削減が期待できます。
- 標準的なパーフュージョン攪拌型培養槽を用いた種培養と比較し、バッチあたりのコストを25%削減します。
- 力価を30%以上向上させます。
- アップストリームの種培養期間を短縮
Biostat STR®による生産性と柔軟性の向上
ザルトリウス社のBiostat STR®バイオリアクターは、統合されたXCell ATF®技術により灌流プロセスを簡素化します。ATFを超濾過モードで使用することで、生産用バイオリアクター内での細胞の完全な保持が可能となり、濃縮された製品が得られます。これにより、従来のフェドバッチ方式と比較して、はるかに高い力価が実現されます。強化されたシードトレイン内でこの運転モードを導入することで、以下の効果が期待できます:
- 施設のスループットが最大4倍向上
- バッチあたりの生産性向上(最大4倍の力価向上)
- バッチ時間の短縮によるさらなるスループットと柔軟性の向上
Media Management
Ensuring Media Quality and Stability Is Critical for Intensification
Concentrated Fed-Batch requires a larger amount of cell culture medium than in standard fed-batch. Ensuring consistent cell culture performance in intensified processes is dependent on the media design, quality, and stability. Consumables and equipment to handle these volumes are equally essential to support a smooth process flow. A reliable filter to avoid contamination and remove mycoplasma is also critical for successful intensified processes.
The Flexsafe® Pro Mixer Saves Time Without Compromising Control
Flexsafe® Pro Mixer is an efficient, single-use mixer for 50 L up to 3000 L. It provides the high levels of control that are essential for cGMP biomanufacturing.
- Saves time with fast powder dissolution
- Preserves quality of shear sensitive fluids through low shear mixing and high torque
- Designed for rapid set-up and efficient changeover (in less than 10 minutes) reducing risk of damage and loss from product mishandling
- Includes full material characterization and safety evaluation
Single-Use Bags
2D Single-Use Bags
Storage to Support the Media Volume Required for Concentrated Fed-Batch
With concentrated fed-batch running in perfusion mode, there is a greater demand for cell culture medium. It is for this reason that consumables and equipment to handle these volumes are essential for a smooth process flow. Sartorius Flexsafe® 2D Single-Use Bags ensure the stability and integrity of the medium, allowing excellent, reproducible cell growth. The superior strength of the polyethylene film reduces risk of damage, while its flexibility enables consistent performance and easy handling.
3D Single-Use Bags
Self-Deploying and Filling 3D Single-Use Bags for Pallet Tanks
Liquid medium must be prepared and safely stored to be ready whenever it is needed. To meet the significant cell culture media requirements of concentrated fed-batch and support smooth process flow, larger scale liquid storage is often required. Flexsafe® 3D Single-Use Bags, designed for storage applications in pallet tanks, are easy to use and allow excellent and reproducible cell growth. With a self-deploying accessory, the bag can be filled with no operator manipulation.These ready-to-use bags have “plug and play” compatibility with Sartorius Biostat STR® bioreactors.
Ensuring Quality and Safety of Cell Culture Medium
Companies are looking more and more for virus removal filters for media for risk mitigation purposes. Virosart® Media filters, provide high virus clearance safety and show superior capacities for media used for mAbs and recombinant proteins.
Cost Efficient Media Filtration with High Mycoplasma Retention
Sartopore® 2 XLM sterile filters are used for cost-efficient media filtration with outstanding high throughput. They feature a 30% larger surface area and optimized pre-filter layers which results in a higher flow rate and higher throughput. The Sartopore® 2 XLM is an advanced 0.1 µm rated filter with high mycoplasma retention.
Process Data Analytics
Assurance Through Multivariate Data Analytics
Customers rely on enhanced data analytics for process intensification. This is due to the increased complexity of processes, operations, and data, as well as the total cost of risk. Multivariate Data Analysis (MVDA) extracts meaningful information from large data sets. It enables scale-up and batch-to-batch comparison investigations to improve the quality, safety, and efficacy of a drug product.
SIMCA® Ensures Process Health and Manufacturing Success
SIMCA® is an advanced data analytics solution to gain actionable information from large quantities of data, ensuring consistent processes and product quality. SIMCA® analyzes incoming raw materials and determines how they may affect cell growth, viability, productivity, and quality – which is critical for concentrated fed-batch. This robust multivariate engine will compare batch-to-batch quality and identify sources of any variation, accessible through an intuitive interface and easy-to-read interactive visualizations.
SIMCA®-Online Integrates Real-Time Monitoring and Controls
Multivariate real-time monitoring is important, especially in intensified processes. While SIMCA® creates a digital fingerprint of a process as reference, SIMCA®-online uses real-time measurements to assess operations based on this fingerprint – essentially verifying if the current batch fingerprint matches or not. If there are derivations from the reference, the process can be adapted immediately. This automated corrective action keeps quality consistent, maximizes efficiency and reduces costs.