Solutions for Lateral Flow Assays & In Vitro Diagnostics Kit (IVD) Manufacturers
Diagnostic OEM Membranes, Tailored Lab Instruments & Consumables, Upstream Process Development & Characterization Tools
Sartorius solutions streamline diagnostic industry development and quality control workflows. Our expertise in biotherapeutics and personalized offerings can help you:
- Design a standardized sample preparation protocol
- Achieve optimal performance characteristics on lateral flow rapid tests with consistent and homogeneous UniSart® membranes
- Monitor and trace your critical Quality Control parameters
- Accelerate your analyte discovery steps, development and production
Trust Sartorius to help you meet aggressive milestones and tough standards for performance in the Diagnostic industry.
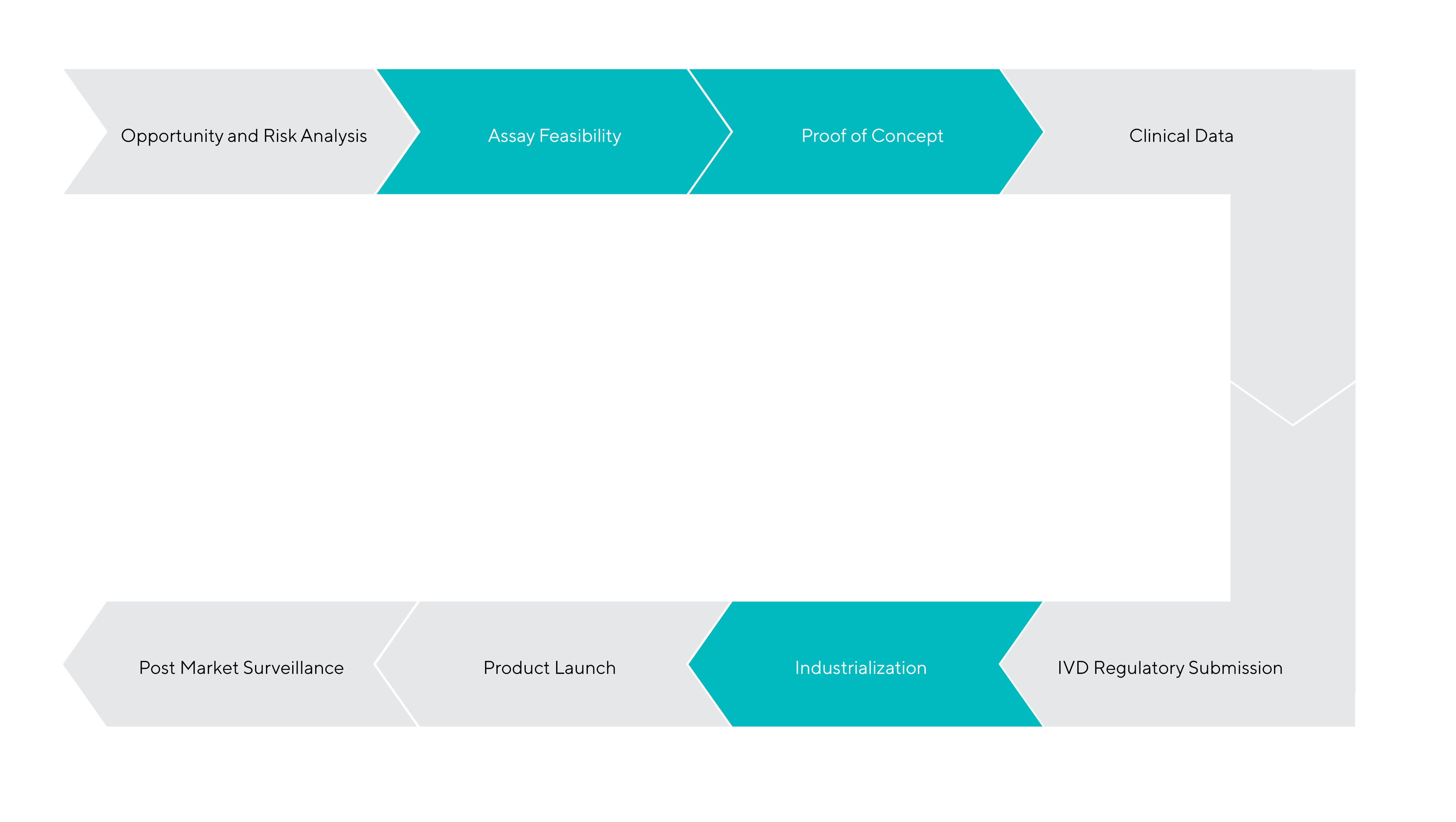
Development of a Novel IVD Test
Featured Products
Membranes for In Vitro Diagnostics, Microarray & OEM
Unisart® nitrocellulose membranes are designed to serve as the perfect solid phase for binding proteins on test and control lines, as well as to facilitate the transportation of the sample-conjugate complex in diagnostic lateral flow immunoassays (LFIAs).
- Inter- and intra-lot consistency to maintain production parameters between lots
- Reduced scrap rates with smooth, defect-free membrane surfaces
- High-sensitivity membrane allows detection of minimal biomarker concentrations
- Complete lot traceability over the entire casting process, from mixing of raw materials to the final slit roll
- ISO certified manufacturing, 100% controlled by backlight inspection
Label-free Bio-Layer Interferometry (BLI) technology
The Octet® R8 system utilizes Sartorius’ label-free Bio-Layer Interferometry (BLI) technology, enabling direct detection of specific proteins and other biomarkers. It can be used for a wide range of analyses including kinetic analysis, quantitation of IgGs and other proteins, immunoassay development, quality analysis, epitope binning/mapping and ligand binding assays.
- Excellent alternative to assays performed using time- and labor-intensive methods such as ELISA and HPLC.
- Eight channels can be used independently to measure samples for screening purposes or in tandem, pairing the sample read with a dedicated reference for high-quality kinetic characterization.
- Monitors binding events in real- time measuring binding association (ka) and dissociation (kd) kinetics, and affinity constants (KD).
- Optional 21 CFR Part 11 software available along with installation and qualification (IQOQ) and performance qualification (PQ) kits.
- Human antibody titer using Protein A biosensors can be determined in minutes.
Applications for IVD Manufacturers & Lateral Flow Assays
Customized Preparation of Patient Samples
Diagnostic testing requires repetitive but critical preparation steps. IVD systems should be compact with high throughput to minimize low-value tasks in clinical labs. Sartorius offers application-specific instruments and consumables for secured diagnosis (Dx) and precise sample preparation, or for easy integration in your systems. These include:
- Liquid handling instruments with embedded workflows to standardize your strictly defined protocol
- High-purity pipette tips free of DNase, RNase and endotoxins - available in bespoke packaging formats
- General Laboratory Use (GLU) ultrafiltration devices with volumes and membranes tailored optimizing human specimen sample preparation
- Dispensing heads for easy integration into robotic micro-volume dispensing solutions
- Weigh cells designed for integration into automated systems to control and monitor filling routines from 1 µL to 8
Related:
Publication: "The Impact of IVD Regulations on Manufacturing"
Application Note: "Urine Protein Concentration with Vivaspin®"
Infographic: Strategies for Improving Lab Ergonomics by Using the Right Pipette
Guide: Lab Ultrafiltration Troubleshooting
Guide: Pre-Validation of Ultrafiltration Devices for General Lab Use
Membranes for Lateral Flow Assays
Lateral flow assays (LFAs) are commonly used for rapid detection of pathogens, toxins, and environmental contaminants. Nitrocellulose UniSart® membranes are the ideal substrate for diagnostic tests and are widely used in strip or flow-through devices. Sartorius offers a microfluidic design with hydrophobic barriers for multiparameter assays.
Sartorius’ broad variety of UniSart® membranes allows selection of the best performing characteristics for your device.
- Highly efficient matrix with reliable capillary speed and protein binding developed to offer high reproducible readouts and protein binding
- Precise traceability and consistent membrane quality for a reliable supply, from many m² of one batch down to the few cm² of a lateral strip
- Smooth, and defect-free surface allows for a consistent dispensing of sharp capture and control reagents lines
Related:
Publication: "The Emergence of Lateral Flow Assays in Diagnostics"
Video: How to Select a UniSart® Diagnostic Membrane for Lateral Flow Assays
Video: How to Measure Physical Properties of UniSart® Diagnostic Membranes
Video: How to Improve Diagnostic Membrane Performance via Dispenser Setup
Quality Control of Target Analyte & Product Development
Inadequate sample preparation tools can lead to false-positive or false-negative identification of interferences. Microbial contamination encountered during production steps and detected late can affect analytical performance, efficiency and the time-to-market. All chemicals used in the test should be carefully monitored from incoming, in-process and packing materials to finished goods.
Sartorius can help monitor and trace critical parameters with:
- Octet® Label-free Bio-Layer Interferometry (BLI) technology enabling direct detection of specific receptor proteins, ligands, antibodies and other analyte specific reagents (ASRs)
- Cubis® II Pharma Compliant Weighing and sample management solutions, designed to follow data integrity principles and regulatory compliance
- Rapid detection of mycoplasma, bacteria and fungi in your product in just three hours with ATMP Microsart® qPCR kits
- Microsart® family for microbial enumeration, bioburden testing, with touch-free membrane transfer
Related:
Poster: Advancing the Use of Octet® Label-Free Bio-Layer Interferometry (BLI)
Whitepaper: Benefits of Using Octet® BLI Systems in IVD Product Development
Whitepaper: Cubis® II Pharma Compliant by Design
Analyte Specific Reagent (ASR) Discovery & Optimization
Speed is crucial in a competitive environment, and the COVID-19 pandemic has highlighted the importance of being first-to-market with a novel analyte-specific reagent (ASR).
Sartorius offers in-house expertise to optimize analyte performance, discover new binding abilities, and improve processes for more efficient operations. With a strong background in biologics molecule development, we can help accelerate your antibody development.
- Perform preclinical evaluations of drug treatments by utilizing 3D patient-derived cultures and identify new diagnostic antibodies with Incucyte® Live-Cell Analysis Systems
- Search the best binding pairs among different nucleoproteins concentration with Octet® (BLI) Detection Systems for Label-Free detection
- Achieve cultivation and media optimization for optimal growth and protein production with Ambr® 15 Cell Culture Bioreactor System
Related:
Brochure: Insightful Solutions for Biologics Drug Discovery with a focus on Monoclonal Antibodies (mAbs)
Application Note: Ultrapure Water to Precise 2D-PAGE Results
Sartorius IVD Manufacturing Solutions Offer:
Our international team of qualified experts understand what is needed when developing a novel diagnostic test. Your partnership with Sartorius is strictly confidential and backed by personalized support throughout the entire project.
OEM supplies are vital in diagnostic development. Sartorius products are developed, produced and distributed to ISO 9001 Quality Management System standards. They meet stringent quality control standards throughout all manufacturing steps and are backed by quality assurance certificates that guarantee conformity to specifications.
In additional to expertise in the production of reliable nitrocellulose membranes, Sartorius has also developed a comprehensive portfolio of scalable solutions for manufacturing and development of biomarkers, from media to bioproduction and capture. Finally, our compliant QC solutions help you ensure that your product is safe, effective and pure.
Medical Device and IVD Resources
Instant Access
Related Blog Posts
Frequently Asked Questions
In vitro molecular or nucleic acid-based diagnostics identify genetic, infectious, viral diseases or tumors by targeting and amplifying a specific nucleic acid fragment among millions of other DNA/RNA fragments.
During RNA preparation, cDNA synthesis and RT-PCR are pipetting-intensive protocols. It is crucial that the tips used are free of RNase and DNase with a barrier-filter that protect patient samples from cross-contamination.
The role of the nitrocellulose membranes in a lateral flow immunoassay (LFIA) is highly critical. The membrane acts as the main motor of the test, drawing the samples and conjugates from one end of the strip to the other end. Concurrently, the sample-conjugate complex can be captured by reagents that are pre-bound to the test and control lines.
Forward and reverse protein arrays are used in many multiparameter clinical diagnostic tests. The multiplicity of protein spots (in picoliter range), displayed in an intricate pattern on the slide surface, allow looking at various interactions simultaneously. The UniSart® 3D slide is a special glass slide that is coated with a thin microporous nitrocellulose membrane. The pad on the slide is a three-dimensional microporous nitrocellulose membrane, which shows high protein affinity and binding capacity in all microarray applications.
In Vitro Diagnostics (IVD) refers to a broad range of tests performed to detect diseases and various conditions and can be used to monitor an individual’s overall health. The diagnostics are done on blood, tissue, saliva or urina samples. They vary widely in targeted molecules and are aimed at the detection of low expressing biomarkers. The targeted diagnostic biomarker detects or confirms the presence of a disease or condition of interest, or identifies an individual with a subtype of the disease. In Vitro diagnostics products typically include systems and analyte specific reagents such as receptor proteins, ligands or antibodies that are designed with high specificity and sensitivity to detect and quantify changes in the targeted biomarker level as a function of the disease state
Sartorius has had a number of very successful collaborations for pipettes and pipette tips, and always welcomes new requests. Our team of development scientists and engineers will work closely with you to discuss the feasibility of creating a custom product that meets your needs (e.g. custom aliquots, private labelling, specific multi-dispensing application).
Please complete the form and we'll contact you to arrange a call to discuss needs, timeline and budget.C3 needs (e.g. custom aliquots, private labelling, specific multi-dispensing application)
Please complete the form and we'll contact you to arrange a call to discuss your needs, timeline, and budget.
Key applications of filtration membranes in non-implantable medical devices include sterilizing filtration, filtration of protein-containing solutions, mycoplasma reduction, gas filtration, venting, sterile insufflation or suction and acting as a barrier against contaminants or particles. Sartorius offers membranes that can meet your specific requirements. To proceed, please fill in the provided form. Be prepared to answer questions regarding application, minimum quantity, packaging, forecast, regulations, compliance, roadmap and expected delays. Rest assured, your confidentiality will be respected throughout this process.
Vivaspin® and Vivaspin® Turbo are designed to potentially achieve concentration factors of 100-fold or more. However, the maximum concentration factor that can be achieved for a specific sample depends on several factors. These include the type of sample, amount of protein, chosen molecular weight cut-off (MWCO) and duration of centrifugation. the amount of protein, the chosen molecular weight cut-off (MWCO), and the duration of centrifugation.
Optimizing Diagnostic Sample Prep Workflows Using Ultrafiltration
No. All Vivaspin® units are designed with built-in dead stops ranging from 5 μL to 350 μL. This feature ensures the target molecule is not lost by concentrating it to dryness. Moreover, each device - especially the Vivaspin® Turbo with its unique angular dead stop - is designed to allow easy aspiration of every last microliter of the concentrated sample using standard pipette tips.
Optimizing Diagnostic Sample Prep Workflows Using Ultrafiltration
If you're considering incorporating Vivaspin® GLU units into your diagnostic kit or workflow, we provide a pre-validation document to guide you through the process. Initially, we recommend conducting a total protein (TP) recovery test after sample concentration. This can be done using either the concentration factor test or the sample dilution test. To ensure reproducibility, it's suggested that labs calculate the average total protein recovery from 20 different human specimens. For precision, the average total protein recovery should be determined five times using the same human specimen. Some labs may choose to calculate these values for each batch of ultrafilters used.
Optimizing Diagnostic Sample Prep Workflows Using Ultrafiltration
If your lab processes urine samples, you might be worried about pH changes due to certain diseases. We have tested Vivaspin® for resistance within the pH range of 4.5–8 using human urine samples. However, we recommend that you conduct your own tests and validate these ultrafilters based on your specific needs and requirements. For further guidance, please refer to the relevant instructions or contact our technical support team.
Optimizing Diagnostic Sample Prep Workflows Using Ultrafiltration
One of the major advantages of the Octet® BLI system is its ability to rapidly screen media for optimal conditions during assay development. This assists developers in finding the best buffer for antibody immobilization and optimizing the pre-treatment of sample pads. It's important to note that not all antibodies perform well in a specific buffer system. Octet® BLI systems can also be utilized for rapid buffer matrix screening affinity assays, where a single analyte concentration is tested for binding in various matrices. These matrices may be spiked with different excipients to determine optimal binding conditions. Additionally, the characteristics of reference antibodies can be stored and used for comparison with samples stored over time, which can help monitor antibody stability, among other things.
Octet® BLI systems are available in a variety of throughput configurations, capable of running 1 to 96 samples in parallel, depending on the model. For laboratories with lower throughput needs, the Octet N1 or R2 would be suitable. However, for substantial throughput requirements or for full GxP compliance, users should consider the Octet R8, RH16 or RH96. These models have the capability to process 8 to 96 samples concurrently.
Sample volumes for Octet® BLI experiments can vary depending on the specific instrument model. However, sample concentrations are dependent on affinity constants of the molecules being studied. The higher the affinity, the lower the concentration of sample required. Octet® BLI systems have the capability to measure up to pM affinity, where the highest analyte concentrations used are in the low nM range.
Yes, it is indeed possible to measure aptamers using Octet® BLI systems. As long as one molecule can be immobilized onto a biosensor surface, the binding of another molecule can be assessed. Molecular weights are more critical than the sample type, and the instrument is capable of measuring the interaction of molecules ranging in size from peptides to bacteria and viruses.
LFAs offer numerous advantages, including the ability to deliver reliable results quickly, which can be qualitative, semi-quantitative or quantitative. They are also more cost-effective compared to laboratory diagnostics and are readily available in drug stores and pharmacies. Their user-friendly nature makes them convenient for all end-users, as they require no special training. Moreover, LFAs can be utilized anywhere, independent of laboratory infrastructure. These characteristics contribute to the success of rapid tests.
Unisart® CN 180 DX combines a high protein binding capacity with superior sensitivity, making it ideal for critical applications such as infectious diseases (e.g., HIV and malaria). The increased inner surface area of the membrane allows for more protein binding. The combination of increased protein binding and decreased capillary speed results in enhanced signal intensity. This makes Unisart® CN 180 DX the membrane of choice for critical applications where sensitivity is of utmost importance.
One of the most crucial steps in diagnostic applications is the precise and specific detection of the target analyte or biomarker. The quality and efficiency of sample preparation directly impact the sensitivity, specificity and reliability of subsequent detection steps. Sample preparation enhances diagnostic testing success by eliminating interfering substances, improving detection specificity and accuracy and facilitating concentration and enrichment of the analyte of interest. It also aids in preserving and stabilizing the patient sample and target analyte during storage and transportation. Sartorius provides specific and adapted sample preparation devices or support by integrating a dedicated workflow into our pipettes to ensure the pre-diagnostic steps.
Yes. It is possible to deposit droplets at any position on the membrane due to SCIENION's unique non-contact sciPICO technology. You can dispense one spot after the other along the channel's direction. As a result of the dual high-resolution cameras, the sciFLEXARRAYER system provides full control over drop generation and target positioning, allowing printing into very small cavities, such as the 6 channels of the Unisart StructSure membrane from Sartorius. The spot area on the membrane can be minimized so that the sample will interact with every spot without any signal reduction, which is typically observed at Multiplex LFA in line format due to detector depletion.
Any type of sample is printable, such as proteins, glycans, DNA, cells or organic solvents. Different sample types bring unique dispensing challenges, which can be addressed by choosing the right PDC (piezo dispense capillary) type with a suitable coating and the right dispensing mode within the sciFLEXARRAYER systems. Furthermore, the choice of the correct print buffer from a selection of sciBUFFERs ensures uniform spot morphology while maintaining the functionality of the biomolecules for downstream assay requirements. Our experts will advise you on the optimal print conditions for your specific sample.
There are no limitations on the printing targets that can be used. If the target does not permit effective immobilization of the sample, SCIENION offers surface activation to enhance binding. We also provide microarray consumables such as sciCHIPs with surface-functionalizations such as epoxy-, amino- or aldehyde groups, which can be used to effectively bind proteins, glycans, and DNA. Our team will be glad to assist you in choosing the most appropriate target and surface for your product.